Local oxidative stress expansion through endothelial cells--a key role for gap junction intercellular communication
- PMID: 22911831
- PMCID: PMC3402439
- DOI: 10.1371/journal.pone.0041633
Local oxidative stress expansion through endothelial cells--a key role for gap junction intercellular communication
Abstract
Background: Major circulation pathologies are initiated by oxidative insult expansion from a few injured endothelial cells to distal sites; this possibly involves mechanisms that are important to understanding circulation physiology and designing therapeutic management of myocardial pathologies. We tested the hypothesis that a localized oxidative insult of endothelial cells (ECs) propagates through gap junction inter-cellular communication (GJIC).
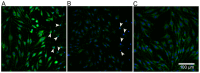
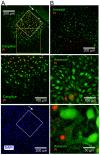
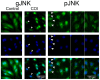
Methodology/principal findings: Cultures comprising the bEnd.3 cell line, that have been established and recognized as suitable for examining communication among ECs, were used to study the propagation of a localized oxidative insult to remote cells. Spatially confined near infrared illumination of parental or genetically modified bEnd.3 cultures, pretreated with the photosensitizer WST11, generated O(2)•(-) and •OH radicals in the illuminated cells. Time-lapse fluorescence microscopy, utilizing various markers, and other methods, were used to monitor the response of non-illuminated bystander and remote cells. Functional GJIC among ECs was shown to be mandatory for oxidative insult propagation, comprising de-novo generation of reactive oxygen and nitrogen species (ROS and RNS, respectively), activation and nuclear translocation of c-Jun N-terminal kinase, followed by massive apoptosis in all bystander cells adjacent to the primarily injured ECs. The oxidative insult propagated through GJIC for many hours, over hundreds of microns from the primary photogeneration site. This wave is shown to be limited by intracellular ROS scavenging, chemical GJIC inhibition or genetic manipulation of connexin 43 (a key component of GJIC).
Conclusion/significance: Localized oxidative insults propagate through GJIC between ECs, while stimulating de-novo generation of ROS and RNS in bystander cells, thereby driving the insult's expansion.
Conflict of interest statement
Figures





References
-
- Bogdan C, R llinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Current opinion in immunology. 2000;12:64–76. - PubMed
-
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science's STKE. 2009;2:ra45. - PubMed
-
- Ruiz-Gines J, Lopez-Ongil S, Gonzalez-Rubio M, Gonzalez-Santiago L, Rodriguez-Puyol M, et al. Reactive oxygen species induce proliferation of bovine aortic endothelial cells. Journal of cardiovascular pharmacology. 2000;35:109. - PubMed
-
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. - PubMed
-
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circulation research. 2000;87:179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

