Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation
- PMID: 22911892
- PMCID: PMC4442093
- DOI: 10.1002/stem.1207
Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation
Abstract
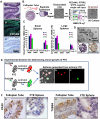
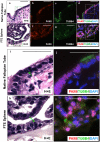
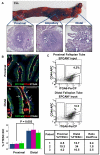
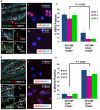
The reproductive role of the fallopian tube is to transport the sperm and egg. The tube is positioned to act as a bridge between the ovary where the egg is released and the uterus where implantation occurs. Throughout reproductive years, the fallopian tube epithelium undergoes repetitive damage and regeneration. Although a reservoir of adult epithelial stem cells must exist to replenish damaged cells, they remain unidentified. Here, we report isolation of a subset of basally located human fallopian tube epithelia (FTE) that lack markers of ciliated (β-tubulin; TUBB4) or secretory (PAX8) differentiated cells. These undifferentiated cells expressed cell surface antigens: epithelial cell adhesion molecule, CD44, and integrin α 6. This FTE subpopulation was fivefold enriched for cells capable of clonal growth and self-renewal suggesting that they contain the FTE stem-like cells (FTESCs). A twofold enrichment of the FTESC was found in the distal compared to the proximal end of the tube. The distal fimbriated end of the fallopian tube is a well-characterized locus for initiation of serous carcinomas. An expansion of the cells expressing markers of FTESC was detected in tubal intraepithelial carcinomas and in fallopian tubes from patients with invasive serous cancer. These findings suggest that FTESC may play a role in the initiation of serous tumors. Characterization of these stem-like cells will provide new insight into how the FTE regenerate, respond to injury, and may initiate cancer.
Copyright © 2012 AlphaMed Press.
Figures



References
-
- Critoph FN, Dennis KJ. Ciliary activity in the human oviduct. Obstet Gynecol Surv. 1977;32:602–603. - PubMed
-
- Mahmood T, Saridogan E, Smutna S, et al. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod. 1998;13:2991–2994. - PubMed
-
- Lyons RA, Djahanbakhch O, Mahmood T, et al. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum Reprod. 2002;17:584–588. - PubMed
-
- Leese HJ, Tay JI, Reischl J, et al. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001;121:339–346. - PubMed
-
- Crow J, Amso NN, Lewin J, et al. Morphology and ultrastructure of fallopian tube epithelium at different stages of the menstrual cycle and menopause. Hum Reprod. 1994;9:2224–2233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

