Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis
- PMID: 22912398
- PMCID: PMC3437887
- DOI: 10.1073/pnas.1207488109
Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis
Abstract
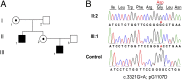
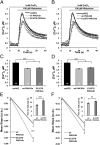
Ca(2+) in neurons is vital to processes such as neurotransmission, neurotoxicity, synaptic development, and gene expression. Disruption of Ca(2+) homeostasis occurs in brain aging and in neurodegenerative disorders. Membrane transporters, among them the calmodulin (CaM)-activated plasma membrane Ca(2+) ATPases (PMCAs) that extrude Ca(2+) from the cell, play a key role in neuronal Ca(2+) homeostasis. Using X-exome sequencing we have identified a missense mutation (G1107D) in the CaM-binding domain of isoform 3 of the PMCAs in a family with X-linked congenital cerebellar ataxia. PMCA3 is highly expressed in the cerebellum, particularly in the presynaptic terminals of parallel fibers-Purkinje neurons. To study the effects of the mutation on Ca(2+) extrusion by the pump, model cells (HeLa) were cotransfected with expression plasmids encoding its mutant or wild-type (wt) variants and with the Ca(2+)-sensing probe aequorin. The mutation reduced the ability of the PMCA3 pump to control the cellular homeostasis of Ca(2+). It significantly slowed the return to baseline of the Ca(2+) transient induced by an inositol-trisphosphate (InsP(3))-linked plasma membrane agonist. It also compromised the ability of the pump to oppose the influx of Ca(2+) through the plasma membrane capacitative channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. - PubMed
-
- Falchetto R, Vorherr T, Brunner J, Carafoli E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J Biol Chem. 1991;266:2930–2936. - PubMed
-
- Burette A, Weinberg RJ. Perisynaptic organization of plasma membrane calcium pumps in cerebellar cortex. J Comp Neurol. 2007;500:1127–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

