Cross-talk between the fat body and brain regulates insect developmental arrest
- PMID: 22912402
- PMCID: PMC3437847
- DOI: 10.1073/pnas.1212879109
Cross-talk between the fat body and brain regulates insect developmental arrest
Abstract
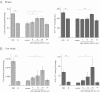
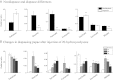
Developmental arrest, a critical component of the life cycle in animals as diverse as nematodes (dauer state), insects (diapause), and vertebrates (hibernation), results in dramatic depression of the metabolic rate and a profound extension in longevity. Although many details of the hormonal systems controlling developmental arrest are well-known, we know little about the interactions between metabolic events and the hormones controlling the arrested state. Here, we show that diapause is regulated by an interplay between blood-borne metabolites and regulatory centers within the brain. Gene expression in the fat body, the insect equivalent of the liver, is strongly suppressed during diapause, resulting in low levels of tricarboxylic acid (TCA) intermediates circulating within the blood, and at diapause termination, the fat body becomes activated, releasing an abundance of TCA intermediates that act on the brain to stimulate synthesis of regulatory peptides that prompt production of the insect growth hormone ecdysone. This model is supported by our success in breaking diapause by injecting a mixture of TCA intermediates and upstream metabolites. The results underscore the importance of cross-talk between the brain and fat body as a regulator of diapause and suggest that the TCA cycle may be a checkpoint for regulating different forms of animal dormancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish.Am J Physiol Regul Integr Comp Physiol. 2016 Jun 1;310(11):R1193-211. doi: 10.1152/ajpregu.00250.2015. Epub 2016 Apr 6. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27053646 Free PMC article. Review.
-
Identification, characterization, and developmental regulation of two storage proteins in the bamboo borer Omphisa fuscidentalis.J Insect Physiol. 2008 Jan;54(1):62-76. doi: 10.1016/j.jinsphys.2007.08.003. Epub 2007 Aug 10. J Insect Physiol. 2008. PMID: 17869264
-
Expression of the early-late gene encoding the nuclear receptor HR3 suggests its involvement in regulating the vitellogenic response to ecdysone in the adult mosquito.Mol Cell Endocrinol. 2000 Feb 25;160(1-2):25-37. doi: 10.1016/s0303-7207(99)00253-1. Mol Cell Endocrinol. 2000. PMID: 10715536
-
Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug, Pyrrhocoris apterus.J Insect Physiol. 2008 Jan;54(1):77-88. doi: 10.1016/j.jinsphys.2007.08.004. Epub 2007 Aug 15. J Insect Physiol. 2008. PMID: 17880995
-
Steroid regulation of C. elegans diapause, developmental timing, and longevity.Curr Top Dev Biol. 2013;105:181-212. doi: 10.1016/B978-0-12-396968-2.00007-5. Curr Top Dev Biol. 2013. PMID: 23962843 Review.
Cited by
-
Quantitative Proteomic and Transcriptomic Analyses of Metabolic Regulation of Adult Reproductive Diapause in Drosophila suzukii (Diptera: Drosophilidae) Females.Front Physiol. 2019 Apr 4;10:344. doi: 10.3389/fphys.2019.00344. eCollection 2019. Front Physiol. 2019. PMID: 31019467 Free PMC article.
-
Upregulation of Insulin and Ecdysone Signaling in Relation to Diapause Termination in Bombyx mori Eggs Exposed to 5 °C.Insects. 2024 Dec 12;15(12):989. doi: 10.3390/insects15120989. Insects. 2024. PMID: 39769591 Free PMC article.
-
Changes in Energy Metabolism Trigger Pupal Diapause Transition of Bactrocera minax After 20-Hydroxyecdysone Application.Front Physiol. 2019 Oct 30;10:1288. doi: 10.3389/fphys.2019.01288. eCollection 2019. Front Physiol. 2019. PMID: 31736767 Free PMC article.
-
Global metabolomic analyses of the hemolymph and brain during the initiation, maintenance, and termination of pupal diapause in the cotton bollworm, Helicoverpa armigera.PLoS One. 2014 Jun 13;9(6):e99948. doi: 10.1371/journal.pone.0099948. eCollection 2014. PLoS One. 2014. PMID: 24926789 Free PMC article.
-
New roles for old actors, ROS and PRMT1.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):10810-10812. doi: 10.1073/pnas.1715062114. Epub 2017 Oct 2. Proc Natl Acad Sci U S A. 2017. PMID: 28973953 Free PMC article. No abstract available.
References
-
- Zhang Q, Denlinger DL. Dynamics of diapause hormone and prothoracicotropic hormone transcript expression at diapause termination in pupae of the corn earworm, Helicoverpa zea. Peptides. 2012;34:120–126. - PubMed
-
- Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Insect Molecular Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol 3. Amsterdam: Elsevier; 2005. pp. 615–650.
-
- Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113–127. - PubMed
-
- Hu PJ. 2007. The C. elegans Research Community, WormBook. Available at http://www.wormbook.org. Accessed August 8, 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

