Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors
- PMID: 22912406
- PMCID: PMC3437860
- DOI: 10.1073/pnas.1212264109
Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors
Abstract
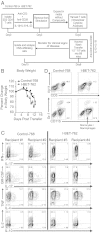
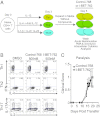
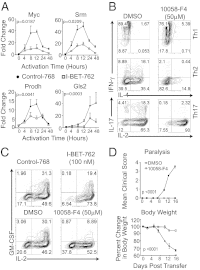
Bromodomain-containing proteins bind acetylated lysine residues on histone tails and are involved in the recruitment of additional factors that mediate histone modifications and enable transcription. A compound, I-BET-762, that inhibits binding of an acetylated histone peptide to proteins of the bromodomain and extra-terminal domain (BET) family, was previously shown to suppress the production of proinflammatory proteins by macrophages and block acute inflammation in mice. Here, we investigated the effect of short-term treatment with I-BET-762 on T-cell function. Treatment of naïve CD4(+) T cells with I-BET-762 during the first 2 d of differentiation had long-lasting effects on subsequent gene expression and cytokine production. Gene expression analysis revealed up-regulated expression of several antiinflammatory gene products, including IL-10, Lag3, and Egr2, and down-regulated expression of several proinflammatory cytokines including GM-CSF and IL-17. The short 2-d treatment with I-BET-762 inhibited the ability of antigen-specific T cells, differentiated under Th1 but not Th17 conditions in vitro, to induce pathogenesis in an adoptive transfer model of experimental autoimmune encephalomyelitis. The suppressive effects of I-BET-762 on T-cell mediated inflammation in vivo were accompanied by decreased recruitment of macrophages, consistent with decreased GM-CSF production by CNS-infiltrating T cells. These effects were mimicked by an inhibitor of c-myc function, implicating reduced expression of c-myc and GM-CSF as one avenue by which I-BET-762 suppresses the inflammatory functions of T cells. Our study demonstrates that inhibiting the functions of BET-family proteins during early T-cell differentiation causes long-lasting suppression of the proinflammatory functions of Th1 cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

