A canonical model of multistability and scale-invariance in biological systems
- PMID: 22912567
- PMCID: PMC3415415
- DOI: 10.1371/journal.pcbi.1002634
A canonical model of multistability and scale-invariance in biological systems
Abstract
Multistability and scale-invariant fluctuations occur in a wide variety of biological organisms from bacteria to humans as well as financial, chemical and complex physical systems. Multistability refers to noise driven switches between multiple weakly stable states. Scale-invariant fluctuations arise when there is an approximately constant ratio between the mean and standard deviation of a system's fluctuations. Both are an important property of human perception, movement, decision making and computation and they occur together in the human alpha rhythm, imparting it with complex dynamical behavior. Here, we elucidate their fundamental dynamical mechanisms in a canonical model of nonlinear bifurcations under stochastic fluctuations. We find that the co-occurrence of multistability and scale-invariant fluctuations mandates two important dynamical properties: Multistability arises in the presence of a subcritical Hopf bifurcation, which generates co-existing attractors, whilst the introduction of multiplicative (state-dependent) noise ensures that as the system jumps between these attractors, fluctuations remain in constant proportion to their mean and their temporal statistics become long-tailed. The simple algebraic construction of this model affords a systematic analysis of the contribution of stochastic and nonlinear processes to cortical rhythms, complementing a recently proposed biophysical model. Similar dynamics also occur in a kinetic model of gene regulation, suggesting universality across a broad class of biological phenomena.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
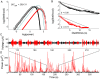
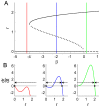
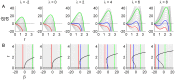

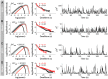
 . C: Phase portraits of example integrations. Black/red indicates inward/outward flow. D: Time series of example integrations. E: Corresponding power PDFs.
. C: Phase portraits of example integrations. Black/red indicates inward/outward flow. D: Time series of example integrations. E: Corresponding power PDFs.  is the discrete-time analytic signal of r (i.e. the amplitude envelope of r).
is the discrete-time analytic signal of r (i.e. the amplitude envelope of r).
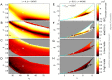
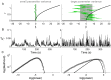
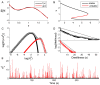
 . C: Corresponding PDF.
. C: Corresponding PDF.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

