Influences of chloride and hypochlorite on neutrophil extracellular trap formation
- PMID: 22912772
- PMCID: PMC3418225
- DOI: 10.1371/journal.pone.0042984
Influences of chloride and hypochlorite on neutrophil extracellular trap formation
Abstract
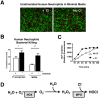
Background: The release by neutrophils of DNA-based extracellular traps (NETs) is a recently recognized innate immune phenomenon that contributes significantly to control of bacterial pathogens at tissue foci of infection. NETs have also been implicated in the pathogenesis of non-infectious diseases such as small vessel vasculitis, lupus and cystic fibrosis lung disease. Reactive oxygen species (ROS) are important mediators of NET generation (NETosis). Neutrophils with reduced ROS production, such as those from patients with chronic granulomatous disease or myeloperoxidase (MPO) deficiency, produce fewer NETs in response to inflammatory stimuli. To better understand the roles of various ROS in NETosis, we explore the role of MPO, its substrates chloride ion (Cl(-)) and hydrogen peroxide (H(2)O(2)), and its product hypochlorite (HOCl) in NETosis.
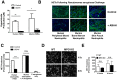
Findings: In human peripheral blood neutrophils, pharmacologic inhibition of MPO decreased NETosis. Absence of extracellular Cl(-), a substrate for MPO, also reduced NETosis. While exogenous addition of H(2)O(2) and HOCl stimulated NETosis, only exogenous HOCl could rescue NETosis in the setting of MPO inhibition. Neither pharmacological inhibition nor genetic deletion of MPO in murine neutrophils blocked NETosis, in contrast to findings in human neutrophils.
Conclusions: Our results pinpoint HOCl as the key ROS involved in human NETosis. This finding has implications for understanding innate immune function in diseases in which Cl(-) homeostasis is disturbed, such as cystic fibrosis. Our results also reveal an example of significant species-specific differences in NET phenotypes, and the need for caution in extrapolation to humans from studies of murine NETosis.
Conflict of interest statement
Figures



References
-
- Nauseef WM (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219: 88–102. - PubMed
-
- Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5: 577–582. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

