Quantification of lung fibrosis and emphysema in mice using automated micro-computed tomography
- PMID: 22912805
- PMCID: PMC3418271
- DOI: 10.1371/journal.pone.0043123
Quantification of lung fibrosis and emphysema in mice using automated micro-computed tomography
Abstract
Background: In vivo high-resolution micro-computed tomography allows for longitudinal image-based measurements in animal models of lung disease. The combination of repetitive high resolution imaging with fully automated quantitative image analysis in mouse models of lung fibrosis lung benefits preclinical research. This study aimed to develop and validate such an automated micro-computed tomography analysis algorithm for quantification of aerated lung volume in mice; an indicator of pulmonary fibrosis and emphysema severity.
Methodology: Mice received an intratracheal instillation of bleomycin (n = 8), elastase (0.25 U elastase n = 9, 0.5 U elastase n = 8) or saline control (n = 6 for fibrosis, n = 5 for emphysema). A subset of mice was scanned without intervention, to evaluate potential radiation-induced toxicity (n = 4). Some bleomycin-instilled mice were treated with imatinib for proof of concept (n = 8). Mice were scanned weekly, until four weeks after induction, when they underwent pulmonary function testing, lung histology and collagen quantification. Aerated lung volumes were calculated with our automated algorithm.
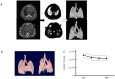
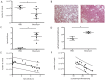
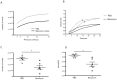
Principal findings: Our automated image-based aerated lung volume quantification method is reproducible with low intra-subject variability. Bleomycin-treated mice had significantly lower scan-derived aerated lung volumes, compared to controls. Aerated lung volume correlated with the histopathological fibrosis score and total lung collagen content. Inversely, a dose-dependent increase in lung volume was observed in elastase-treated mice. Serial scanning of individual mice is feasible and visualized dynamic disease progression. No radiation-induced toxicity was observed. Three-dimensional images provided critical topographical information.
Conclusions: We report on a high resolution in vivo micro-computed tomography image analysis algorithm that runs fully automated and allows quantification of aerated lung volume in mice. This method is reproducible with low inherent measurement variability. We show that it is a reliable quantitative tool to investigate experimental lung fibrosis and emphysema in mice. Its non-invasive nature has the unique benefit to allow dynamic 4D evaluation of disease processes and therapeutic interventions.
Conflict of interest statement
Figures



References
-
- Ley B, Collard HR, King TE Jr. (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2011;183(4):431–40. Epub 2010/10/12. - PubMed
-
- Moore BB, Hogaboam CM (2008) Murine models of pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2008;294(2):L152-60. Epub 2007/11/13. - PubMed
-
- Lederlin M, Ozier A, Montaudon M, Begueret H, Ousova O, et al.. (2010) Airway remodeling in a mouse asthma model assessed by in-vivo respiratory-gated micro-computed tomography. Eur Radiol. 2010;20(1):128–37. Epub 2009/08/18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

