Broad expression analysis of human ANTXR1/TEM8 transcripts reveals differential expression and novel splizce variants
- PMID: 22912819
- PMCID: PMC3422265
- DOI: 10.1371/journal.pone.0043174
Broad expression analysis of human ANTXR1/TEM8 transcripts reveals differential expression and novel splizce variants
Erratum in
- PLoS One. 2012; 7(10): 10.1371/annotation/cebf633f-19e7-496b-b370-c0f1b1aea888
Abstract
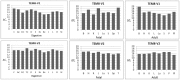
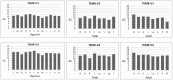
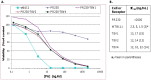
Tumor endothelial marker 8 (TEM8; ANTXR1) is one of two anthrax toxin receptors; the other is capillary morphogenesis gene 2 protein (CMG2; ANTXR2). TEM8 shows enhanced expression in certain tumor endothelia, and is thought to be a player in tumor vasculature formation. However, a comprehensive expression profile of individual TEM8 variants in normal or cancerous tissues is lacking. In this work we carried out an extensive analysis of all splice variants of human TEM8 in 12 digestive tissues, and 8 each fetal and adult tissues, 6 of them cognate pairs. Using variant-specific primers, we first ascertained the status of full-length transcripts by nested PCR. We then carried out quantitative analysis of each transcript by real-time PCR. Three splice variants of TEM8 were reported before, two single-pass integral membrane forms (V1 and V2) and one secreted (V3). Our analysis has revealed two new variants, one encoding a membrane-bound form of the receptor and the other secreted, which we have designated V4 and V5, respectively. All tissues had V1, V2, V3, and V4, but only prostate had V5. Real-time PCR revealed that all variants are present at different levels in various tissues. V3 appeared the most abundant of all. To ascertain its functionality for anthrax toxin, we expressed the newly identified form V4 in a receptor-negative host cell, and included V1 and V2 for comparison. Cytotoxicity, toxin binding, and internalization assays showed V4 to be as efficient a receptor as V1 and V2.
Conflict of interest statement
Figures



Similar articles
-
Selection of anthrax toxin protective antigen variants that discriminate between the cellular receptors TEM8 and CMG2 and achieve targeting of tumor cells.J Biol Chem. 2007 Mar 30;282(13):9834-9845. doi: 10.1074/jbc.M611142200. Epub 2007 Jan 24. J Biol Chem. 2007. PMID: 17251181 Free PMC article.
-
The structure of tumor endothelial marker 8 (TEM8) extracellular domain and implications for its receptor function for recognizing anthrax toxin.PLoS One. 2010 Jun 18;5(6):e11203. doi: 10.1371/journal.pone.0011203. PLoS One. 2010. PMID: 20585457 Free PMC article.
-
ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis' three sites of entry: implications for the pathogenesis of anthrax infection.Am J Physiol Cell Physiol. 2005 Jun;288(6):C1402-10. doi: 10.1152/ajpcell.00582.2004. Epub 2005 Feb 2. Am J Physiol Cell Physiol. 2005. PMID: 15689409
-
TEM8 in Oncogenesis: Protein Biology, Pre-Clinical Agents, and Clinical Rationale.Cells. 2023 Nov 14;12(22):2623. doi: 10.3390/cells12222623. Cells. 2023. PMID: 37998358 Free PMC article. Review.
-
Converging physiological roles of the anthrax toxin receptors.F1000Res. 2019 Aug 12;8:F1000 Faculty Rev-1415. doi: 10.12688/f1000research.19423.1. eCollection 2019. F1000Res. 2019. PMID: 31448094 Free PMC article. Review.
Cited by
-
Tumor endothelial marker 8 promotes cancer progression and metastasis.Oncotarget. 2018 Jul 10;9(53):30173-30188. doi: 10.18632/oncotarget.25734. eCollection 2018 Jul 10. Oncotarget. 2018. PMID: 30046396 Free PMC article.
-
Regulatory mechanisms of anthrax toxin receptor 1-dependent vascular and connective tissue homeostasis.Matrix Biol. 2015 Mar;42:56-73. doi: 10.1016/j.matbio.2014.12.002. Epub 2015 Jan 5. Matrix Biol. 2015. PMID: 25572963 Free PMC article.
-
Fell-Muir Lecture: Regulatory mechanisms of skeletal and connective tissue development and homeostasis - lessons from studies of human disorders.Int J Exp Pathol. 2016 Aug;97(4):296-302. doi: 10.1111/iep.12198. Epub 2016 Sep 1. Int J Exp Pathol. 2016. PMID: 27581728 Free PMC article. Review.
-
Emerging Immunotherapies against Novel Molecular Targets in Breast Cancer.Int J Mol Sci. 2021 Feb 28;22(5):2433. doi: 10.3390/ijms22052433. Int J Mol Sci. 2021. PMID: 33670942 Free PMC article. Review.
-
The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer.Cancers (Basel). 2023 Mar 4;15(5):1597. doi: 10.3390/cancers15051597. Cancers (Basel). 2023. PMID: 36900394 Free PMC article. Review.
References
-
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, et al. (2000) Genes expressed in human tumor endothelium. Science 289: 1197–1202. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA (2001) Identification of the cellular receptor for anthrax toxin. Nature 414: 225–229. - PubMed
-
- Liu S, Leppla SH (2003) Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalizations. J Biol Chem 278: 5227–5234. - PubMed
-
- Scobie HM, Young JA (2005) Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol 8(1): 106–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

