Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease
- PMID: 22912851
- PMCID: PMC3422338
- DOI: 10.1371/journal.pone.0043310
Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease
Abstract
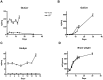
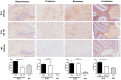
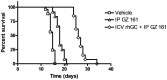
Neuropathic Gaucher disease (nGD), also known as type 2 or type 3 Gaucher disease, is caused by a deficiency of the enzyme glucocerebrosidase (GC). This deficiency impairs the degradation of glucosylceramide (GluCer) and glucosylsphingosine (GluSph), leading to their accumulation in the brains of patients and mouse models of the disease. These accumulated substrates have been thought to cause the severe neuropathology and early death observed in patients with nGD and mouse models. Substrate accumulation is evident at birth in both nGD mouse models and humans affected with the most severe type of the disease. Current treatment of non-nGD relies on the intravenous delivery of recombinant human glucocerebrosidase to replace the missing enzyme or the administration of glucosylceramide synthase inhibitors to attenuate GluCer production. However, the currently approved drugs that use these mechanisms do not cross the blood brain barrier, and thus are not expected to provide a benefit for the neurological complications in nGD patients. Here we report the successful reduction of substrate accumulation and CNS pathology together with a significant increase in lifespan after systemic administration of a novel glucosylceramide synthase inhibitor to a mouse model of nGD. To our knowledge this is the first compound shown to cross the blood brain barrier and reduce substrates in this animal model while significantly enhancing its lifespan. These results reinforce the concept that systemically administered glucosylceramide synthase inhibitors could hold enhanced therapeutic promise for patients afflicted with neuropathic lysosomal storage diseases.
Conflict of interest statement
Figures



References
-
- Barranger JA GE (1989) Glucosylceramide lipidosis: Gaucher disease. In: Scriver CR BA, Sly WS, Valle D, editor. The Metabolic Basis of Inherited Disease. New York: McGraw-Hill. 1677–1698.
-
- Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, et al. (2003) Phenotypic continuum in neuronopathic gaucher disease: an intermediate phenotype between type 2 and type 3. The Journal of Pediatrics 143: 273–276. - PubMed
-
- Nilsson O SL (1982) Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J Neurochem 39: 709–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

