A novel highly potent therapeutic antibody neutralizes multiple human chemokines and mimics viral immune modulation
- PMID: 22912856
- PMCID: PMC3422223
- DOI: 10.1371/journal.pone.0043332
A novel highly potent therapeutic antibody neutralizes multiple human chemokines and mimics viral immune modulation
Abstract
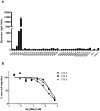
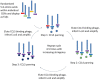
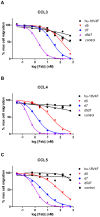
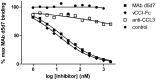
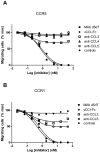
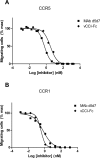
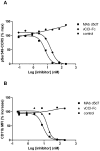
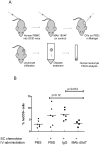
Chemokines play a key role in leukocyte recruitment during inflammation and are implicated in the pathogenesis of a number of autoimmune diseases. As such, inhibiting chemokine signaling has been of keen interest for the development of therapeutic agents. This endeavor, however, has been hampered due to complexities in the chemokine system. Many chemokines have been shown to signal through multiple receptors and, conversely, most chemokine receptors bind to more than one chemokine. One approach to overcoming this complexity is to develop a single therapeutic agent that binds and inactivates multiple chemokines, similar to an immune evasion strategy utilized by a number of viruses. Here, we describe the development and characterization of a novel therapeutic antibody that targets a subset of human CC chemokines, specifically CCL3, CCL4, and CCL5, involved in chronic inflammatory diseases. Using a sequential immunization approach, followed by humanization and phage display affinity maturation, a therapeutic antibody was developed that displays high binding affinity towards the three targeted chemokines. In vitro, this antibody potently inhibits chemotaxis and chemokine-mediated signaling through CCR1 and CCR5, primary chemokine receptors for the targeted chemokines. Furthermore, we have demonstrated in vivo efficacy of the antibody in a SCID-hu mouse model of skin leukocyte migration, thus confirming its potential as a novel therapeutic chemokine antagonist. We anticipate that this antibody will have broad therapeutic utility in the treatment of a number of autoimmune diseases due to its ability to simultaneously neutralize multiple chemokines implicated in disease pathogenesis.
Conflict of interest statement
Figures
Similar articles
-
MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4.J Leukoc Biol. 2005 Jul;78(1):167-77. doi: 10.1189/jlb.0404237. Epub 2005 Apr 14. J Leukoc Biol. 2005. PMID: 15831559
-
Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice.Bone. 2010 Apr;46(4):1122-30. doi: 10.1016/j.bone.2009.12.030. Epub 2010 Jan 4. Bone. 2010. PMID: 20053385
-
CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts.J Bone Miner Res. 2004 Dec;19(12):2065-77. doi: 10.1359/JBMR.040910. Epub 2004 Sep 20. J Bone Miner Res. 2004. PMID: 15537451
-
CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy.Immunol Lett. 2020 Jan;217:91-115. doi: 10.1016/j.imlet.2019.11.007. Epub 2019 Nov 17. Immunol Lett. 2020. PMID: 31747563 Review.
-
Regulation of chemokine activity by posttranslational modification.Pharmacol Ther. 2008 Nov;120(2):197-217. doi: 10.1016/j.pharmthera.2008.08.006. Epub 2008 Aug 28. Pharmacol Ther. 2008. PMID: 18793669 Review.
Cited by
-
A strategy to discover decoy chemokine ligands with an anti-inflammatory activity.Sci Rep. 2015 Oct 7;5:14746. doi: 10.1038/srep14746. Sci Rep. 2015. PMID: 26442456 Free PMC article.
-
Contribution of viral mimics of cellular genes to KSHV infection and disease.Viruses. 2014 Sep 19;6(9):3472-86. doi: 10.3390/v6093472. Viruses. 2014. PMID: 25243371 Free PMC article. Review.
-
Clinical and therapeutic implications of sex steroid hormone receptor status in urothelial bladder cancer.Indian J Urol. 2020 Jul-Sep;36(3):171-178. doi: 10.4103/iju.IJU_320_19. Epub 2020 Jul 1. Indian J Urol. 2020. PMID: 33082631 Free PMC article. Review.
-
Targeting CCL5 in inflammation.Expert Opin Ther Targets. 2013 Dec;17(12):1439-60. doi: 10.1517/14728222.2013.837886. Epub 2013 Oct 3. Expert Opin Ther Targets. 2013. PMID: 24090198 Free PMC article. Review.
-
Synthetic phage for tissue regeneration.Mediators Inflamm. 2014;2014:192790. doi: 10.1155/2014/192790. Epub 2014 May 27. Mediators Inflamm. 2014. PMID: 24991085 Free PMC article. Review.
References
-
- Sallusto F, Baggiolini M (2008) Chemokines and leukocyte traffic. Nat Immunol 9: 949–952. - PubMed
-
- Rossi D, Zlotnik A (2000) The biology of chemokines and their receptors. Annu Rev Immunol 18: 217–242. - PubMed
-
- Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423: 356–361. - PubMed
-
- Sorensen TL, Ransohoff RM (1998) Etiology and pathogenesis of multiple sclerosis. Semin Neurol 18: 287–294. - PubMed
-
- Coyle PK (2010) The role of natalizumab in the treatment of multiple sclerosis. Am J Manag Care 16: S164–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

