Evaluation of environmental safety concentrations of DMSA Coated Fe2O3-NPs using different assay systems in nematode Caenorhabditis elegans
- PMID: 22912902
- PMCID: PMC3422352
- DOI: 10.1371/journal.pone.0043729
Evaluation of environmental safety concentrations of DMSA Coated Fe2O3-NPs using different assay systems in nematode Caenorhabditis elegans
Abstract
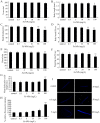
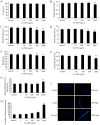
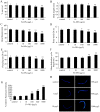
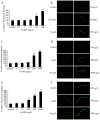
Dimercaptosuccinic acid (DMSA) coating improves the uptake efficiency presumably by engendering the Fe(2)O(3)-NPs. In the present study, we investigated the possible environmental safety concentrations of Fe(2)O(3)-NPs using different assay systems in nematode Caenorhabditis elegans with lethality, development, reproduction, locomotion behavior, pharyngeal pumping, defecation, intestinal autofluorescence and reactive oxygen species (ROS) production as the endpoints. After exposure from L4-larvae for 24-hr, DMSA coated Fe(2)O(3)-NPs at concentrations more than 50 mg/L exhibited adverse effects on nematodes. After exposure from L1-larvae to adult, DMSA coated Fe(2)O(3)-NPs at concentrations more than 500 μg/L had adverse effects on nematodes. After exposure from L1-larvae to day-8 adult, DMSA coated Fe(2)O(3)-NPs at concentrations more than 100 μg/L resulted in the adverse effects on nematodes. Accompanied with the alterations of locomotion behaviors, ROS production was pronouncedly induced by exposure to DMSA coated Fe(2)O(3)-NPs in the examined three assay systems, and the close associations of ROS production with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, or intestinal autofluorescence in nematodes exposed to DMSA coated Fe(2)O(3)-NPs were confirmed by the linear regression analysis. Moreover, mutations of sod-2 and sod-3 genes, encoding Mn-SODs, showed more susceptible properties than wild-type when they were used for assessing the DMSA coated Fe(2)O(3)-NPs-induced toxicity, and the safety concentrations for DMSA coated Fe(2)O(3)-NPs should be defined as concentrations lower than 10 μg/L in sod-2 and sod-3 mutant nematodes.
Conflict of interest statement
Figures
Similar articles
-
Molecular control of TiO₂-NPs toxicity formation at predicted environmental relevant concentrations by Mn-SODs proteins.PLoS One. 2012;7(9):e44688. doi: 10.1371/journal.pone.0044688. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973466 Free PMC article.
-
Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans.J Hazard Mater. 2012 Jun 15;219-220:221-30. doi: 10.1016/j.jhazmat.2012.03.083. Epub 2012 Apr 6. J Hazard Mater. 2012. PMID: 22521136
-
Comparison of toxicities from three metal oxide nanoparticles at environmental relevant concentrations in nematode Caenorhabditis elegans.Chemosphere. 2013 Jan;90(3):1123-31. doi: 10.1016/j.chemosphere.2012.09.019. Epub 2012 Oct 9. Chemosphere. 2013. PMID: 23062833
-
Toxicity comparison of nanopolystyrene with three metal oxide nanoparticles in nematode Caenorhabditis elegans.Chemosphere. 2020 Apr;245:125625. doi: 10.1016/j.chemosphere.2019.125625. Epub 2019 Dec 16. Chemosphere. 2020. PMID: 31855754
-
Ecotoxicity of Caenorhabditis elegans following a step and repeated chronic exposure to tetrabromobisphenol A.Ecotoxicol Environ Saf. 2019 Mar;169:273-281. doi: 10.1016/j.ecoenv.2018.10.113. Epub 2018 Nov 16. Ecotoxicol Environ Saf. 2019. PMID: 30453175
Cited by
-
Materials and toxicological approaches to study metal and metal-oxide nanoparticles in the model organism Caenorhabditis elegans.Mater Horiz. 2017 Sep 1;4(5):719-746. doi: 10.1039/C7MH00166E. Epub 2017 May 3. Mater Horiz. 2017. PMID: 29057078 Free PMC article.
-
Morphological Analysis of Reticuloendothelial System in Capuchin Monkeys (Sapajus spp.) after Meso-2,3-Dimercaptosuccinic Acid (DMSA) Coated Magnetic Nanoparticles Administration.PLoS One. 2015 Nov 11;10(11):e0140233. doi: 10.1371/journal.pone.0140233. eCollection 2015. PLoS One. 2015. PMID: 26559061 Free PMC article.
-
Sublethal Effects of Ionic and Nanogold on the Nematode Caenorhabditis elegans.J Toxicol. 2018 Nov 1;2018:6218193. doi: 10.1155/2018/6218193. eCollection 2018. J Toxicol. 2018. PMID: 30515209 Free PMC article.
-
Adverse effects from clenbuterol and ractopamine on nematode Caenorhabditis elegans and the underlying mechanism.PLoS One. 2014 Jan 21;9(1):e85482. doi: 10.1371/journal.pone.0085482. eCollection 2014. PLoS One. 2014. PMID: 24465573 Free PMC article.
-
High concentration of vitamin E decreases thermosensation and thermotaxis learning and the underlying mechanisms in the nematode Caenorhabditis elegans.PLoS One. 2013 Aug 12;8(8):e71180. doi: 10.1371/journal.pone.0071180. eCollection 2013. PLoS One. 2013. PMID: 23951104 Free PMC article.
References
-
- Hood E (2004) Nanotechnology: looking as we leap. Environ Health Perspect 112: A470–749. - PubMed
-
- Pisanic II TR, Blackwell JD, Shubayev VI, Finones, Jin S (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28: 2572–2581. - PubMed
-
- Karlsson HL, Gustafsson J, Cronholm P, Moller L (2009) Size-dependent toxicity of metal oxide particles – a comparison between nano- and micrometer size. Toxicol Lett 188: 112–118. - PubMed
-
- Wang X, Tang M, Zhang T, Yang L, Xia T, et al. (2007) Oxidative injury of mgahetic ferric oxide nanoparticles to peritoneal macrophage in mice. J Clin Rehab Tissue Engineer Res 11: 2575–2577.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

