Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6
- PMID: 22912904
- PMCID: PMC3422344
- DOI: 10.1371/journal.pone.0043768
Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6
Abstract
Background: Mesothelial cell injury plays an important role in peritoneal fibrosis. Present clinical therapies aimed at alleviating peritoneal fibrosis have been largely inadequate. Mesenchymal stem cells (MSCs) are efficient for repairing injuries and reducing fibrosis. This study was designed to investigate the effects of MSCs on injured mesothelial cells and peritoneal fibrosis.
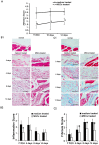
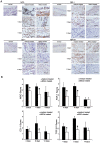
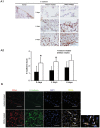
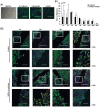
Methodology/principal findings: Rat bone marrow-derived MSCs (5 × 10(6)) were injected into Sprague-Dawley (SD) rats via tail vein 24 h after peritoneal scraping. Distinct reductions in adhesion formation; infiltration of neutrophils, macrophage cells; number of fibroblasts; and level of transforming growth factor (TGF)-β1 were found in MSCs-treated rats. The proliferation and repair of peritoneal mesothelial cells in MSCs-treated rats were stimulated. Mechanically injured mesothelial cells co-cultured with MSCs in transwells showed distinct increases in migration and proliferation. In vivo imaging showed that MSCs injected intravenously mainly accumulated in the lungs which persisted for at least seven days. No apparent MSCs were observed in the injured peritoneum even when MSCs were injected intraperitoneally. The injection of serum-starved MSCs-conditioned medium (CM) intravenously reduced adhesions similar to MSCs. Antibody based protein array of MSCs-CM showed that the releasing of TNFα-stimulating gene (TSG)-6 increased most dramatically. Promotion of mesothelial cell repair and reduction of peritoneal adhesion were produced by the administration of recombinant mouse (rm) TSG-6, and were weakened by TSG-6-RNA interfering.
Conclusions/significance: Collectively, these results indicate that MSCs may attenuate peritoneal injury by repairing mesothelial cells, reducing inflammation and fibrosis. Rather than the engraftment, the secretion of TSG-6 by MSCs makes a major contribution to the therapeutic benefits of MSCs.
Conflict of interest statement
Figures
Similar articles
-
Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6.Stem Cell Res Ther. 2012 Dec 6;3(6):51. doi: 10.1186/scrt142. Stem Cell Res Ther. 2012. PMID: 23217986 Free PMC article.
-
Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling.Kidney Int. 2013 Aug;84(2):297-307. doi: 10.1038/ki.2013.81. Epub 2013 Mar 13. Kidney Int. 2013. PMID: 23486522 Free PMC article.
-
Serum-Free Medium Enhances the Immunosuppressive and Antifibrotic Abilities of Mesenchymal Stem Cells Utilized in Experimental Renal Fibrosis.Stem Cells Transl Med. 2018 Dec;7(12):893-905. doi: 10.1002/sctm.17-0284. Epub 2018 Sep 30. Stem Cells Transl Med. 2018. PMID: 30269426 Free PMC article.
-
Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition.Int J Mol Sci. 2020 Jun 10;21(11):4158. doi: 10.3390/ijms21114158. Int J Mol Sci. 2020. PMID: 32532126 Free PMC article. Review.
-
Mesenchymal Stromal Cells Derived Conditioned Medium in Pulmonary Fibrosis: A Systematic Review and Meta-analysis.Arch Iran Med. 2020 Dec 1;23(12):870-879. doi: 10.34172/aim.2020.116. Arch Iran Med. 2020. PMID: 33356346
Cited by
-
MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism.Front Immunol. 2021 Apr 30;12:626755. doi: 10.3389/fimmu.2021.626755. eCollection 2021. Front Immunol. 2021. PMID: 33995350 Free PMC article. Review.
-
TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis.Sci Rep. 2020 Feb 7;10(1):2115. doi: 10.1038/s41598-020-58909-4. Sci Rep. 2020. PMID: 32034203 Free PMC article.
-
Adipose-derived mesenchymal stem cells transplantation facilitate experimental peritoneal fibrosis repair by suppressing epithelial-mesenchymal transition.J Nephrol. 2014 Oct;27(5):507-14. doi: 10.1007/s40620-014-0133-5. Epub 2014 Aug 22. J Nephrol. 2014. PMID: 25146164
-
Concise Review: Fat and Furious: Harnessing the Full Potential of Adipose-Derived Stromal Vascular Fraction.Stem Cells Transl Med. 2017 Apr;6(4):1096-1108. doi: 10.1002/sctm.16-0337. Epub 2017 Jan 6. Stem Cells Transl Med. 2017. PMID: 28186685 Free PMC article. Review.
-
Mesenchymal stem cells therapy for acute kidney injury: A systematic review with meta-analysis based on rat model.Front Pharmacol. 2023 Apr 13;14:1099056. doi: 10.3389/fphar.2023.1099056. eCollection 2023. Front Pharmacol. 2023. PMID: 37124211 Free PMC article.
References
-
- Herrick SE, Mutsaers SE (2004) Mesothelial progenitor cells and their potential in tissue engineering. Int J Biochem Cell Biol 36: 621–642. - PubMed
-
- Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, et al. (2007) Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 18: 2004–2013. - PubMed
-
- Saxena R (2008) Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol 23: 695–703. - PubMed
-
- Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, et al. (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479. - PubMed
-
- Williams JD, Craig KJ, von Ruhland C, Topley N, Williams GT (2003) The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int Suppl: S43–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

