Structural analysis of bengamide derivatives as inhibitors of methionine aminopeptidases
- PMID: 22913487
- PMCID: PMC3470909
- DOI: 10.1021/jm3008695
Structural analysis of bengamide derivatives as inhibitors of methionine aminopeptidases
Abstract
Natural-product-derived bengamides possess potent antiproliferative activity and target human methionine aminopeptidases (MetAPs) for their cellular effects. Several derivatives were designed, synthesized, and evaluated as MetAP inhibitors. Here, we present four new X-ray structures of human MetAP1 in complex with the inhibitors. Together with the previous structures of bengamide derivatives with human MetAP2 and tubercular MtMetAP1c, analysis of the interactions of these inhibitors at the active site provides structural basis for further modification of these bengamide inhibitors for improved potency and selectivity as anticancer and antibacterial therapeutics.
Figures




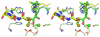

References
-
- Quinoa E, Adamczeski M, Crews P, Bakus GJ. Bengamides, heterocyclic anthelmintics from a Jaspidae marine sponge. J Org Chem. 1986;51:4494–4497.
-
- Kinder FR, Jr., Versace RW, Bair KW, Bontempo JM, Cesarz D, Chen S, Crews P, Czuchta AM, Jagoe CT, Mou Y, Nemzek R, Phillips PE, Tran LD, Wang RM, Weltchek S, Zabludoff S. Synthesis and antitumor activity of ester-modified analogues of bengamide B. J Med Chem. 2001;44:3692–3699. - PubMed
-
- Thale Z, Kinder FR, Bair KW, Bontempo J, Czuchta AM, Versace RW, Phillips PE, Sanders ML, Wattanasin S, Crews P. Bengamides revisited: new structures and antitumor studies. J Org Chem. 2001;66:1733–1741. - PubMed
-
- Phillips PE, Bair KW, Bontempo J, Crews P, Czuchta M,A, Kinder FR, Vattay A, Versace RW, Wang B, Wang J, Wood A, Zabludoff S. Bengamide E arrests cells at the G1/S restriction point and within the G2/M phase of the cell cycle. Proc. Am. Assoc. Cancer Res. 2000;41:59.
-
- Towbin H, Bair KW, DeCaprio JA, Eck MJ, Kim S, Kinder FR, Morollo A, Mueller DR, Schindler P, Song HK, van Oostrum J, Versace RW, Voshol H, Wood J, Zabludoff S, Phillips PE. Proteomics-based target identification: bengamides as a new class of methionine aminopeptidase inhibitors. J Biol Chem. 2003;278:52964–52971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

