Role of µ-opioid receptor reserve and µ-agonist efficacy as determinants of the effects of µ-agonists on intracranial self-stimulation in rats
- PMID: 22914074
- PMCID: PMC3864003
- DOI: 10.1097/FBP.0b013e328358593c
Role of µ-opioid receptor reserve and µ-agonist efficacy as determinants of the effects of µ-agonists on intracranial self-stimulation in rats
Abstract
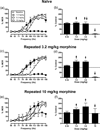
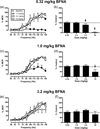
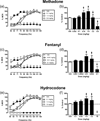
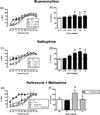
The net effect of µ-opioid receptor agonists on intracranial self-stimulation (ICSS) in rats reflects an integration of rate-increasing and rate-decreasing effects. Previous opioid exposure is associated with tolerance to rate-decreasing effects and the augmented expression of abuse-related rate-increasing effects. This finding was replicated here with morphine. Subsequent studies then tested the hypothesis that opioid agonist-induced rate-decreasing effects require the activation of a larger relative fraction of µ receptors, and hence are more vulnerable to tolerance-associated reductions in receptor density than rate-increasing effects. Two sets of experiments were conducted to test this hypothesis. First, the effects of morphine on ICSS were examined after pretreatment with the irreversible µ antagonist β-funaltrexamine to reduce the density of available µ receptors. Second, effects were examined for a range of µ opioids that varied in relative efficacy at µ receptors. The hypothesis predicted that (a) morphine, after β-funaltrexamine treatment, or (b) low-efficacy µ agonists would mimic the effects of morphine tolerance to produce the reduced expression of rate-decreasing effects and enhanced expression of rate-increasing effects. Neither of these predictions were supported. These results indicate that µ agonist-induced facilitation and depression of ICSS may be mediated by distinct populations of µ receptors that respond differently to regimens of opioid exposure.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures




References
-
- Bardo MT, Gehrke BJ, Shortridge BE, Rauhut AS. Effects of beta-funaltrexamine and naloxonazine on single-trial morphine-conditioned place preference and locomotor activity. Pharmacol Biochem Behav. 2003;74:617–622. - PubMed
-
- Broekkamp CL, Van Den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM. Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol. 1976;36:443–446. - PubMed
-
- Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by mk-801. Brain Res. 1993;620:339–342. - PubMed
-
- Cicero TJ, Dart RC, Inciardi JA, Woody GE, Schnoll S, Munoz A. The development of a comprehensive risk-management program for prescription opioid analgesics: Researched abuse, diversion and addiction-related surveillance (radars) Pain Med. 2007;8:157–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

