STIM proteins: dynamic calcium signal transducers
- PMID: 22914293
- PMCID: PMC3458427
- DOI: 10.1038/nrm3414
STIM proteins: dynamic calcium signal transducers
Abstract
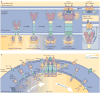
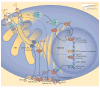
Stromal interaction molecule (STIM) proteins function in cells as dynamic coordinators of cellular calcium (Ca(2+)) signals. Spanning the endoplasmic reticulum (ER) membrane, they sense tiny changes in the levels of Ca(2+) stored within the ER lumen. As ER Ca(2+) is released to generate primary Ca(2+) signals, STIM proteins undergo an intricate activation reaction and rapidly translocate into junctions formed between the ER and the plasma membrane. There, STIM proteins tether and activate the highly Ca(2+)-selective Orai channels to mediate finely controlled Ca(2+) signals and to homeostatically balance cellular Ca(2+). Details are emerging on the remarkable organization within these STIM-induced junctional microdomains and the identification of new regulators and alternative target proteins for STIM.
Conflict of interest statement
The authors declare
Figures


References
-
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

