Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy
- PMID: 22914318
- PMCID: PMC3578982
- DOI: 10.3851/IMP2323
Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy
Abstract
Background: HIV-1-infected individuals with plasma RNA<50 copies/ml on antiretroviral therapy (ART) may have residual, low-level viraemia detectable by PCR assays that are able to detect a single copy of viral RNA (single-copy assay [SCA]). The clinical predictors of residual viraemia in patients on long-term suppressive ART are not yet fully understood.
Methods: We evaluated factors associated with residual viraemia in patients on suppressive ART who underwent screening for a raltegravir intensification trial (ACTG A5244). The screened population was HIV-1-infected adults receiving ART for ≥ 12 months with pre-ART HIV-1 RNA>100,000 copies/ml and on-therapy RNA levels below detection limits of commercial assays for ≥ 6 months.
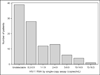
Results: Of 103 patients eligible for analysis, the median age was 46 years and the median duration of viral suppression was 4.8 years. 62% had detectable viraemia (>0.2 copies/ml) by SCA (median 0.2 copies/ml, IQR <0.2-1.8). Younger patients had lower HIV-1 RNA levels than older individuals (r=0.27, P=0.005). Patients with virological suppression on ART for 2 years or less had higher residual viraemia than those with suppression for >2 years (median 2.3 versus 0.2 copies/ml; P=0.016).
Conclusions: Among HIV-1-infected patients with pre-ART HIV-1 RNA>100,000 copies/ml, residual viraemia was detectable in the majority (62%) despite many years of suppressive ART. Higher level viraemia was associated with older age and <2 years of virological suppression on ART. These findings should help in the selection of candidates for clinical trials of interventions designed to eliminate residual viraemia.
Conflict of interest statement
Figures

References
-
- Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS. 1999;13(7):797–804. - PubMed
-
- Walensky R, Paltiel A, Losina E, et al. The Survival Benefits of AIDS Treatment in the United States. J Infect Dis. 2006;194(1):11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI069423/AI/NIAID NIH HHS/United States
- G08 LM008830/LM/NLM NIH HHS/United States
- R01 AI066992/AI/NIAID NIH HHS/United States
- AI 06834/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- 2P30 AI060354-06/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- R01 AI 694722/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

