Phase I safety and immunogenicity evaluations of an alphavirus replicon HIV-1 subtype C gag vaccine in healthy HIV-1-uninfected adults
- PMID: 22914365
- PMCID: PMC3485893
- DOI: 10.1128/CVI.00258-12
Phase I safety and immunogenicity evaluations of an alphavirus replicon HIV-1 subtype C gag vaccine in healthy HIV-1-uninfected adults
Abstract
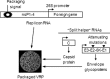
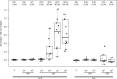
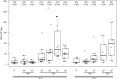
On the basis of positive preclinical data, we evaluated the safety and immunogenicity of an alphavirus replicon HIV-1 subtype C gag vaccine (AVX101), expressing a nonmyristoylated form of Gag, in two double-blind, randomized, placebo-controlled clinical trials in healthy HIV-1-uninfected adults. Escalating doses of AVX101 or placebo were administered subcutaneously to participants in the United States and Southern Africa. Because of vaccine stability issues, the first trial was halted prior to completion of all dose levels and a second trial was implemented. The second trial was also stopped prematurely due to documentation issues with the contract manufacturer. Safety and immunogenicity were evaluated through assessments of reactogenicity, reports of adverse events, and assessment of replication-competent and Venezuelan equine encephalitis (VEE) viremia. Immunogenicity was measured using the following assays: enzyme-linked immunosorbent assay (ELISA), chromium 51 ((51)Cr)-release cytotoxic T lymphocyte (CTL), gamma interferon (IFN-γ) ELISpot, intracellular cytokine staining (ICS), and lymphoproliferation assay (LPA). Anti-vector antibodies were also measured. AVX101 was well tolerated and exhibited only modest local reactogenicity. There were 5 serious adverse events reported during the trials; none were considered related to the study vaccine. In contrast to the preclinical data, immune responses in humans were limited. Only low levels of binding antibodies and T-cell responses were seen at the highest doses. This trial also highlighted the difficulties in developing a novel vector for HIV.
Trial registration: ClinicalTrials.gov NCT00063778 NCT00097838.
Figures



References
-
- Agresti A, Coull BA. 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52:199–256
-
- AlphaVax, Inc 2004. AVX101 clinical investigators brochure. AlphaVax, Inc., Research Triangle Park, NC
-
- Balasuriya UB, et al. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609–1617 - PubMed
-
- Balasuriya UB, et al. 2000. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:10623–10630 - PMC - PubMed
-
- Bernstein DI, et al. 2009. Randomized, double-blind, phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 28:484–493 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI048023/AI/NIAID NIH HHS/United States
- 5-U01-AI-46747/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- 5-U01-AI-47976/AI/NIAID NIH HHS/United States
- 5-U01-AI-47980/AI/NIAID NIH HHS/United States
- U01 AI046747/AI/NIAID NIH HHS/United States
- N01 AI030029/AI/NIAID NIH HHS/United States
- U01 AI047980/AI/NIAID NIH HHS/United States
- 5-U01-AI-48013/AI/NIAID NIH HHS/United States
- U01 AI046703/AI/NIAID NIH HHS/United States
- 5-U01-AI-47985/AI/NIAID NIH HHS/United States
- U01 AI047985/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- 3U01 AIO46747/PHS HHS/United States
- 5 U01 AI550771-02/AI/NIAID NIH HHS/United States
- U01 AI047976/AI/NIAID NIH HHS/United States
- U01 AI048013/AI/NIAID NIH HHS/United States
- 5-U01-AI-48023/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

