Coupling of epithelial Na+ and Cl- channels by direct and indirect activation by serine proteases
- PMID: 22914644
- PMCID: PMC3492828
- DOI: 10.1152/ajpcell.00395.2011
Coupling of epithelial Na+ and Cl- channels by direct and indirect activation by serine proteases
Abstract
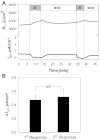
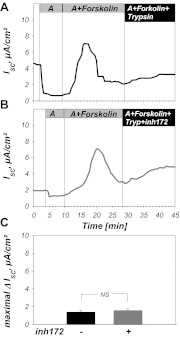
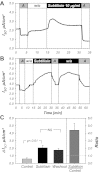
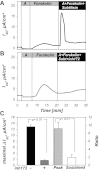
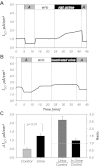
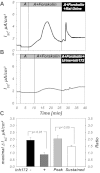
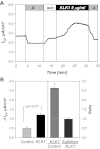
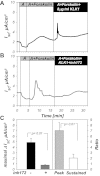
The mammalian collecting duct (CD) is continuously exposed to urinary proteases. The CD expresses an epithelial Na(+) channel (ENaC) that is activated after cleavage by serine proteases. ENaC also exists at the plasma membrane in the uncleaved form, rendering activation by extracellular proteases an important mechanism for regulating Na(+) transport. Many exogenous and a small number of endogenous extracellular serine proteases have been shown to activate the channel. Recently, kallikrein 1 (KLK1) was shown to increase γENaC cleavage in the native CD indicating a possible direct role of this endogenous protease in Na(+) homeostasis. To explore this process, we examined the coordinated effect of this protease on Na(+) and Cl(-) transport in a polarized renal epithelial cell line (Madin-Darby canine kidney). We also examined the role of native urinary proteases in this process. Short-circuit current (I(sc)) was used to measure transport of these ions. The I(sc) exhibited an ENaC-dependent Na(+) component that was amiloride blockable and a cystic fibrosis transmembrane conductance regulator (CFTR)-dependent Cl(-) component that was blocked by inhibitor 172. Apical application of trypsin, an exogenous S1 serine protease, activated I(ENaC) but was without effects on I(CFTR). Subtilisin an exogenous S8 protease that mimics endogenous furin-type proteases activated both currents. A similar activation was also observed with KLK1 and native rat urinary proteases. Activation with urinary proteases occurred within minutes and at protease concentrations similar to those in the CD indicating physiological significance of this process. ENaC activation was irreversible and mediated by enhanced cleavage of γENaC. The activation of CFTR was indirect and likely dependent on activation of an endogenous apical membrane protease receptor. Collectively, these data demonstrate coordinated stimulation of separate Na(+) and Cl(-) transport pathways in renal epithelia by extracellular luminal proteases. They also indicate that baseline urinary proteolytic activity is sufficient to modify Na(+) and Cl(-) transport in these epithelia.
Figures

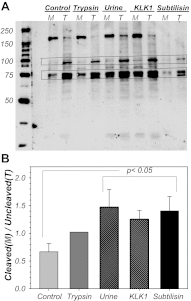

References
-
- Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol 17: 968–976, 2006 - PubMed
-
- Bengrine A, Li J, Hamm LL, Awayda MS. Indirect activation of the epithelial Na+ channel by trypsin. J Biol Chem 282: 26884–26896, 2007 - PubMed
-
- Blazer-Yost BL, Helman SI. The amiloride-sensitive epithelial Na+ channel: binding sites and channel densities. Am J Physiol Cell Physiol 272: C761–C769, 1997 - PubMed
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

