Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice
- PMID: 22914733
- PMCID: PMC3490511
- DOI: 10.1093/hmg/dds348
Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice
Abstract
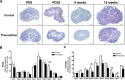
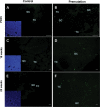
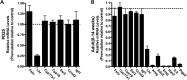
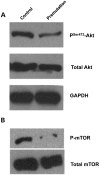
Spontaneous 46,XX primary ovarian insufficiency (POI), also known as 'premature menopause' or 'premature ovarian failure', refers to ovarian dysfunction that results in a range of abnormalities, from infertility to early menopause as the end stage. The most common known genetic cause of POI is the expansion of a CGG repeat to 55-199 copies (premutation) in the 5' untranslated region in the X-linked fragile X mental retardation 1 (FMR1) gene. POI associated with the FMR1 premutation is referred to as fragile X-associated POI (FXPOI). Here, we characterize a mouse model carrying the human FMR1 premutation allele and show that FMR1 premutation RNA can cause a reduction in the number of growing follicles in ovaries and is sufficient to impair female fertility. Alterations in selective serum hormone levels, including FSH, LH and 17β-estradiol, are seen in this mouse model, which mimics findings in humans. In addition, we also find that LH-induced ovulation-related gene expression is specifically altered. Finally, we show that the FMR1 premutation allele can lead to reduced phosphorylation of Akt and mTOR proteins. These results together suggest that FMR1 premutation RNA could cause the POI associated with FMR1 premutation carriers, and the Akt/mTOR pathway may serve as a therapeutic target for FXPOI.
Figures

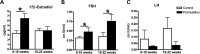
References
-
- Nelson L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009;360:606–614. doi:10.1056/NEJMcp0808697. - DOI - PMC - PubMed
-
- Gallagher J.C. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–571. doi:10.1097/gme.0b013e31804c793d. - DOI - PubMed
-
- Jacobsen B.K., Knutsen S.F., Fraser G.E. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J. Clin. Epidemiol. 1999;52:303–307. doi:10.1016/S0895-4356(98)00170-X. - DOI - PubMed
-
- Mondul A.M., Rodriguez C., Jacobs E.J., Calle E.E. Age at natural menopause and cause-specific mortality. Am. J. Epidemiol. 2005;162:1089–1097. doi:10.1093/aje/kwi324. - DOI - PubMed
-
- Popat V.B., Calis K.A., Vanderhoof V.H., Cizza G., Reynolds J.C., Sebring N., Troendle J.F., Nelson L.M. Bone mineral density in estrogen-deficient young women. J. Clin. Endocrinol. Metab. 2009;94:2277–2283. doi:10.1210/jc.2008-1878. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

