Functional characterization of tissue-specific enhancers in the DLX5/6 locus
- PMID: 22914741
- PMCID: PMC3529576
- DOI: 10.1093/hmg/dds336
Functional characterization of tissue-specific enhancers in the DLX5/6 locus
Abstract
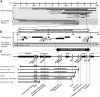
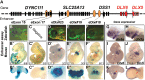
Disruption of distaless homeobox 5 and 6 (Dlx5/6) in mice results in brain, craniofacial, genital, ear and limb defects. In humans, chromosomal aberrations in the DLX5/6 region, some of which do not encompass DLX5/6, are associated with split hand/foot malformation 1 (SHFM1) as well as intellectual disability, craniofacial anomalies and hearing loss, suggesting that the disruption of DLX5/6 regulatory elements could lead to these abnormalities. Here, we characterized enhancers in the DLX5/6 locus whose tissue-specific expression and genomic location along with previously characterized enhancers correlate with phenotypes observed in individuals with chromosomal abnormalities. By analyzing chromosomal aberrations at 7q21, we refined the minimal SHFM1 critical region and used comparative genomics to select 26 evolutionary conserved non-coding sequences in this critical region for zebrafish enhancer assays. Eight of these sequences were shown to function as brain, olfactory bulb, branchial arch, otic vesicle and fin enhancers, recapitulating dlx5a/6a expression. Using a mouse enhancer assay, several of these zebrafish enhancers showed comparable expression patterns in the branchial arch, otic vesicle, forebrain and/or limb at embryonic day 11.5. Examination of the coordinates of various chromosomal rearrangements in conjunction with the genomic location of these tissue-specific enhancers showed a correlation with the observed clinical abnormalities. Our findings suggest that chromosomal abnormalities that disrupt the function of these tissue-specific enhancers could be the cause of SHFM1 and its associated phenotypes. In addition, they highlight specific enhancers in which mutations could lead to non-syndromic hearing loss, craniofacial defects or limb malformations.
Figures
References
-
- Acampora D., Merlo G.R., Paleari L., Zerega B., Postiglione M.P., Mantero S., Bober E., Barbieri O., Simeone A., Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–3809. - PubMed
-
- Jeong J., Li X., McEvilly R.J., Rosenfeld M.G., Lufkin T., Rubenstein J.L. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development. 2008;135:2905–2916. doi:10.1242/dev.019778. - DOI - PMC - PubMed
-
- Suzuki K., Haraguchi R., Ogata T., Barbieri O., Alegria O., Vieux-Rochas M., Nakagata N., Ito M., Mills A.A., Kurita T., et al. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur. J. Hum. Genet. 2008;16:36–44. doi:10.1038/sj.ejhg.5201925. - DOI - PubMed
-
- Robledo R.F., Rajan L., Li X., Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi:10.1101/gad.988402. - DOI - PMC - PubMed
-
- Elliott A.M., Evans J.A. Genotype-phenotype correlations in mapped split hand foot malformation (SHFM) patients. Am. J. Med. Genet. A. 2006;140:1419–1427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HG005058/HG/NHGRI NIH HHS/United States
- 1R01NS079231/NS/NINDS NIH HHS/United States
- R01 HD059862/HD/NICHD NIH HHS/United States
- GM61390/GM/NIGMS NIH HHS/United States
- R01 HG006768/HG/NHGRI NIH HHS/United States
- R01 DK090382/DK/NIDDK NIH HHS/United States
- R01 HG005058/HG/NHGRI NIH HHS/United States
- 1R01HG006768/HG/NHGRI NIH HHS/United States
- 1R01DK090382/DK/NIDDK NIH HHS/United States
- R01 NS079231/NS/NINDS NIH HHS/United States
- U19 GM061390/GM/NIGMS NIH HHS/United States
- R01HD059862/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

