Wnt signaling in vertebrate axis specification
- PMID: 22914799
- PMCID: PMC3579404
- DOI: 10.1101/cshperspect.a007955
Wnt signaling in vertebrate axis specification
Abstract
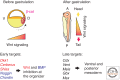
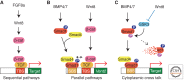
The Wnt pathway is a major embryonic signaling pathway that controls cell proliferation, cell fate, and body-axis determination in vertebrate embryos. Soon after egg fertilization, Wnt pathway components play a role in microtubule-dependent dorsoventral axis specification. Later in embryogenesis, another conserved function of the pathway is to specify the anteroposterior axis. The dual role of Wnt signaling in Xenopus and zebrafish embryos is regulated at different developmental stages by distinct sets of Wnt target genes. This review highlights recent progress in the discrimination of different signaling branches and the identification of specific pathway targets during vertebrate axial development.
Figures

References
-
- Aamar E, Frank D 2004. Xenopus Meis3 protein forms a hindbrain-inducing center by activating FGF/MAP kinase and PCP pathways. Development 131: 153–163 - PubMed
-
- Amaya E, Musci TJ, Kirschner MW 1991. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66: 257–270 - PubMed
-
- Amaya E, Stein PA, Musci TJ, Kirschner MW 1993. FGF signalling in the early specification of mesoderm in Xenopus. Development 118: 477–487 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
