Effect of repetitive lysine-tryptophan motifs on the bactericidal activity of antimicrobial peptides
- PMID: 22914980
- PMCID: PMC3549253
- DOI: 10.1007/s00726-012-1388-6
Effect of repetitive lysine-tryptophan motifs on the bactericidal activity of antimicrobial peptides
Abstract
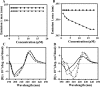
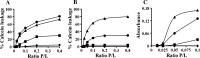
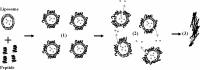
Previous studies identified lysine- and tryptophan-rich sequences within various cationic antimicrobial peptides. In the present study, we synthesized a series of peptides composed of lysine (K)-tryptophan (W) repeats (KW)( n ) (where n equals 2, 3, 4 or 5) with amidation of the C-terminal to increase cationicity. We found that increases in chain length up to (KW)(4) enhanced the peptides' antibacterial activity; (KW)(5) exhibited somewhat less bactericidal activity than (KW)(4). Cytotoxicity, measured as lysis of human red blood cells, also increased with increasing chain length. With (KW)(5), reduced antibacterial activity and increased cytotoxicity correlated with greater hydrophobicity and self-aggregation in the aqueous environment. The peptides acted by inducing rapid collapse of the bacterial transmembrane potential and induction of membrane permeability. The mode of interaction of the peptides and the phosphate groups of lipopolysaccharide was dependent upon the peptides' ability to permeate the membrane. Longer peptides [(KW)(4) and (KW)(5)] but not shorter peptides [(KW)(2) and (KW)(3)] strongly bound and partially inserted into negatively charged, anionic lipid bilayers. These longer peptides also induced membrane permeabilization and aggregation of lipid vesicles. The peptides had a disordered structure in aqueous solution, and only (KW)(4) and (KW)(5) displayed a folded conformation on lipid membranes. Moreover, (KW)(4) destroyed and agglutinated bacterial cells, demonstrating its potential as an antimicrobial agent. Collectively, the results show (KW)(4) to be the most efficacious peptide in the (KW)( n ) series, exhibiting strong antibacterial activity with little cytotoxicity.
Figures





References
-
- Abu-Ghazaleh RI, Gleich GJ, Prendergast FG. Interaction of eosinophil granule major basic protein with synthetic lipid bilayers: a mechanism for toxicity. J Membr Biol. 1992;128:153–164. - PubMed
-
- Andra J, Monreal D, Martinez de Tejada G, Olak C, Brezesinski G, Gomez SS, Goldmann T, Bartels R, Brandenburg K, Moriyon I. Rationale for the design of shortened derivatives of the NK-lysine derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. J Biol Chem. 2007;282:14719–14728. doi: 10.1074/jbc.M608920200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

