Enhancement of ubiquitin conjugation activity reduces intracellular aggregation of V76D mutant γD-crystallin
- PMID: 22915036
- PMCID: PMC3460391
- DOI: 10.1167/iovs.12-9744
Enhancement of ubiquitin conjugation activity reduces intracellular aggregation of V76D mutant γD-crystallin
Abstract
Purpose: The ubiquitin-proteasome pathway (UPP) is an important protein quality control mechanism for selective degradation of abnormal proteins. The objective of this study was to test the hypothesis that enhancement of the UPP capacity could attenuate the accumulation and aggregation of misfolded proteins using V76D-γD-crystallin as a model substrate.
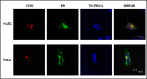
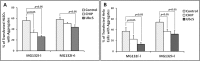
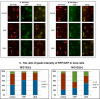
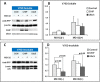
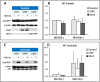
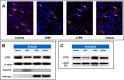
Methods: Wild type (wt) and V76D mutant γD-crystallin were fused to red fluorescence protein (RFP) and expressed in human lens epithelial cells. The cellular distribution of the expressed proteins was compared by fluorescence microscopy. The solubility of wt- and V76D-γD-crystallin was determined by cellular fractionation and Western blotting. Wt-γD-RFP and V76D-γD-RFP were also cotransfected along with a ubiquitin ligase (CHIP) or a ubiquitin-conjugating enzyme (Ubc5) into cells. Levels of wt- and V76D-γD-crystallin, the percentage of transfected cells with aggregates, and aggregate size were quantified and compared among different groups.
Results: Wt-γD-crystallin was evenly distributed in cells, whereas V76D-γD-crystallin formed intracellular aggregates. Eighty percent of wt-γD-crystallin was detected in the soluble fraction, whereas only 7% of V76D-γD-crystallin was soluble. CHIP or Ubc5 coexpression reduced the protein level of V76D-γD and concomitantly its aggregation in transfected cells; these effects could be attenuated by proteasome inhibitor. Mutant CHIP with defect TPR (tetratricopeptide repeat) or U-box domain failed to reduce levels of V76D-γD-crystallin.
Conclusions: Enhancing ubiquitin conjugation activity reduces accumulation and aggregation of V76D-γD-crystallin by promoting its degradation. Upregulation of ubiquitin-conjugating activity could be an effective strategy to maintain lens transparency by eliminating other forms of misfolded proteins.
Conflict of interest statement
Disclosure:
Figures


References
-
- Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta. 2004;1698:131–153 - PubMed
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15:3–16 - PubMed
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 suppl;S10–17 - PubMed
-
- Wistow G, Wyatt K, David L, et al. gammaN-crystallin and the evolution of the betagamma-crystallin superfamily in vertebrates. FEBS J. 2005;272:2276–2291 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

