Microglia proliferation is controlled by P2X7 receptors in a Pannexin-1-independent manner during early embryonic spinal cord invasion
- PMID: 22915101
- PMCID: PMC6703767
- DOI: 10.1523/JNEUROSCI.1042-12.2012
Microglia proliferation is controlled by P2X7 receptors in a Pannexin-1-independent manner during early embryonic spinal cord invasion
Abstract
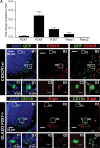
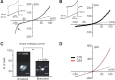
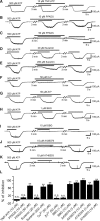
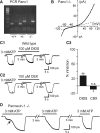
Microglia are known to invade the mammalian spinal cord (SC) at an early embryonic stage. While the mechanisms underlying this early colonization of the nervous system are still unknown, we recently found that it is associated, at least partially, with the ability of microglia to proliferate at the onset of motoneuron developmental cell death and of synaptogenesis in mouse embryo (E13.5). In vitro studies have shown that the proliferation and activation of adult microglia can be influenced by the purinergic ionotropic receptor P2X7 via a coupling with Pannexin-1. By performing patch-clamp recordings in situ using a whole-mouse embryonic SC preparation, we show here that embryonic microglia already express functional P2X7R. P2X7R activation evoked a biphasic current in embryonic microglia, which is supposed to reflect large plasma membrane pore opening. However, although embryonic microglia express pannexin-1, this biphasic current was still recorded in microglia of pannexin-1 knock-out embryos, indicating that it rather reflected P2X7R intrinsic pore dilatation. More important, we found that proliferation of embryonic SC microglia, but not their activation state, depends almost entirely on P2X7R by comparing wild-type and P2X7R-/- embryos. Absence of P2X7R led also to a decrease in microglia density. Pannexin-1-/- embryos did not exhibit any difference in microglial proliferation, showing that the control of embryonic microglial proliferation by P2X7R does not depend on pannexin-1 expression. These results reveal a developmental role of P2X7R by controlling embryonic SC microglia proliferation at a critical developmental state in the SC of mouse embryos.
Figures






Similar articles
-
P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia.Glia. 2014 Apr;62(4):592-607. doi: 10.1002/glia.22628. Epub 2014 Jan 28. Glia. 2014. PMID: 24470356
-
Adaptive phenotype of microglial cells during the normal postnatal development of the somatosensory "Barrel" cortex.Glia. 2013 Oct;61(10):1582-94. doi: 10.1002/glia.22503. Epub 2013 Jul 26. Glia. 2013. PMID: 23893820
-
A central role for P2X7 receptors in human microglia.J Neuroinflammation. 2018 Nov 21;15(1):325. doi: 10.1186/s12974-018-1353-8. J Neuroinflammation. 2018. PMID: 30463629 Free PMC article.
-
Roles of purinergic P2X7 receptor in glioma and microglia in brain tumors.Cancer Lett. 2017 Aug 28;402:93-99. doi: 10.1016/j.canlet.2017.05.004. Epub 2017 May 20. Cancer Lett. 2017. PMID: 28536012 Review.
-
Involvement of microglial P2X7 receptor in pain modulation.CNS Neurosci Ther. 2024 Jan;30(1):e14496. doi: 10.1111/cns.14496. Epub 2023 Nov 10. CNS Neurosci Ther. 2024. PMID: 37950524 Free PMC article. Review.
Cited by
-
Microglia across the lifespan: from origin to function in brain development, plasticity and cognition.J Physiol. 2017 Mar 15;595(6):1929-1945. doi: 10.1113/JP272134. Epub 2016 May 29. J Physiol. 2017. PMID: 27104646 Free PMC article. Review.
-
Astrocyte and neuronal Panx1 support long-term reference memory in mice.bioRxiv [Preprint]. 2023 Jan 18:2023.01.16.524236. doi: 10.1101/2023.01.16.524236. bioRxiv. 2023. Update in: ASN Neuro. 2023 Jan-Dec;15:17590914231184712. doi: 10.1177/17590914231184712. PMID: 36711845 Free PMC article. Updated. Preprint.
-
Origin and Emergence of Microglia in the CNS-An Interesting (Hi)story of an Eccentric Cell.Curr Issues Mol Biol. 2023 Mar 22;45(3):2609-2628. doi: 10.3390/cimb45030171. Curr Issues Mol Biol. 2023. PMID: 36975541 Free PMC article. Review.
-
ATP signaling in brain: release, excitotoxicity and potential therapeutic targets.Cell Mol Neurobiol. 2015 Jan;35(1):1-6. doi: 10.1007/s10571-014-0092-3. Epub 2014 Aug 6. Cell Mol Neurobiol. 2015. PMID: 25096398 Free PMC article. Review.
-
Neonatal Seizures and Purinergic Signalling.Int J Mol Sci. 2020 Oct 22;21(21):7832. doi: 10.3390/ijms21217832. Int J Mol Sci. 2020. PMID: 33105750 Free PMC article. Review.
References
-
- Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem. 2007;101:17–26. - PubMed
-
- Bernier LP, Ase AR, Boué-Grabot E, Séguéla P. P2X4 receptor channels form large noncytolytic pores in resting and activated microglia. Glia. 2012;60:728–737. - PubMed
-
- Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99:745–758. - PubMed
-
- Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
