Netrin-1 attracts axons through FAK-dependent mechanotransduction
- PMID: 22915102
- PMCID: PMC3461192
- DOI: 10.1523/JNEUROSCI.0999-12.2012
Netrin-1 attracts axons through FAK-dependent mechanotransduction
Abstract
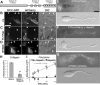
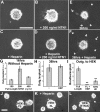
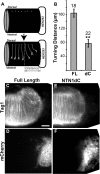
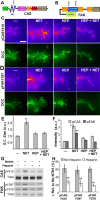
The mechanism by which extracellular cues influence intracellular biochemical cascades that guide axons is important, yet poorly understood. Because of the mechanical nature of axon extension, we explored whether the physical interactions of growth cones with their guidance cues might be involved. In the context of mouse spinal commissural neuron axon attraction to netrin-1, we found that mechanical attachment of netrin-1 to the substrate was required for axon outgrowth, growth cone expansion, axon attraction and phosphorylation of focal adhesion kinase (FAK) and Crk-associated substrate (CAS). Myosin II activity was necessary for traction forces >30 pN on netrin-1. Interestingly, while these myosin II-dependent forces on netrin-1 substrates or beads were needed to increase the kinase activity and phosphorylation of FAK, they were not necessary for netrin-1 to increase CAS phosphorylation. When FAK kinase activity was inhibited, the growth cone's ability to recruit additional adhesions and to generate forces >60 pN on netrin-1 was disrupted. Together, these findings demonstrate an important role for mechanotransduction during chemoattraction to netrin-1 and that mechanical activation of FAK reinforces interactions with netrin-1 allowing greater forces to be exerted.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
