Anti-apoptotic role of caspase-cleaved GAB1 adaptor protein in hepatocyte growth factor/scatter factor-MET receptor protein signaling
- PMID: 22915589
- PMCID: PMC3471683
- DOI: 10.1074/jbc.M112.409797
Anti-apoptotic role of caspase-cleaved GAB1 adaptor protein in hepatocyte growth factor/scatter factor-MET receptor protein signaling
Abstract
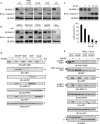
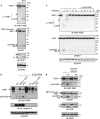
The GRB2-associated binder 1 (GAB1) docking/scaffold protein is a key mediator of the MET-tyrosine kinase receptor activated by hepatocyte growth factor/scatter factor (HGF/SF). Activated MET promotes recruitment and tyrosine phosphorylation of GAB1, which in turn recruits multiple proteins and mediates MET signaling leading to cell survival, motility, and morphogenesis. We previously reported that, without its ligand, MET is a functional caspase target during apoptosis, allowing the generation of a p40-MET fragment that amplifies apoptosis. In this study we established that GAB1 is also a functional caspase target by evidencing a caspase-cleaved p35-GAB1 fragment that contains the MET binding domain. GAB1 is cleaved by caspases before MET, and the resulting p35-GAB1 fragment is phosphorylated by MET upon HGF/SF binding and can interact with a subset of GAB1 partners, PI3K, and GRB2 but not with SHP2. This p35-GAB1 fragment favors cell survival by maintaining HGF/SF-induced MET activation of AKT and by hindering p40-MET pro-apoptotic function. These data demonstrate an anti-apoptotic role of caspase-cleaved GAB1 in HGF/SF-MET signaling.
Figures






Similar articles
-
The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair.Mol Cell Biol. 2001 Aug;21(15):4968-84. doi: 10.1128/MCB.21.15.4968-4984.2001. Mol Cell Biol. 2001. PMID: 11438654 Free PMC article.
-
Degradation of the GAB1 adaptor by the ubiquitin-proteasome pathway hampers HGF/SF-MET signaling.Biochem Biophys Res Commun. 2011 Aug 12;411(4):780-5. doi: 10.1016/j.bbrc.2011.07.024. Epub 2011 Jul 18. Biochem Biophys Res Commun. 2011. PMID: 21782801
-
The involvement of the docking protein Gab1 in mitogenic signalling induced by EGF and HGF in rat hepatocytes.Biochim Biophys Acta. 2013 Dec;1833(12):3286-3294. doi: 10.1016/j.bbamcr.2013.10.004. Epub 2013 Oct 12. Biochim Biophys Acta. 2013. PMID: 24126105
-
HGF/SF-met signaling in the control of branching morphogenesis and invasion.J Cell Biochem. 2003 Feb 1;88(2):408-17. doi: 10.1002/jcb.10358. J Cell Biochem. 2003. PMID: 12520544 Review.
-
Met receptor tyrosine kinase: enhanced signaling through adapter proteins.Oncogene. 2000 Nov 20;19(49):5582-9. doi: 10.1038/sj.onc.1203859. Oncogene. 2000. PMID: 11114738 Review.
Cited by
-
Circulating Apoptotic Signals During Acute and Chronic Exposure to High Altitude in Kyrgyz Population.Front Physiol. 2019 Feb 5;10:54. doi: 10.3389/fphys.2019.00054. eCollection 2019. Front Physiol. 2019. PMID: 30804801 Free PMC article.
References
-
- Schlessinger J. (2000) Cell signaling by receptor-tyrosine kinases. Cell 103, 211–225 - PubMed
-
- Pawson T., Scott J. D. (1997) Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080 - PubMed
-
- Buday L., Tompa P. (2010) Functional classification of scaffold proteins and related molecules. FEBS J. 277, 4348–4355 - PubMed
-
- Holgado-Madruga M., Emlet D. R., Moscatello D. K., Godwin A. K., Wong A. J. (1996) A Grb2-associated docking protein in EGF and insulin receptor signaling. Nature 379, 560–564 - PubMed
-
- Weidner K. M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. (1996) Interaction between Gab1 and the c-Met receptor-tyrosine kinase is responsible for epithelial morphogenesis. Nature 384, 173–176 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

