PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines
- PMID: 22915761
- PMCID: PMC3476734
- DOI: 10.1158/0008-5472.CAN-12-1187
PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines
Abstract
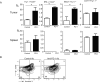
Adoptive cell transfer (ACT) is considered a promising modality for cancer treatment, but despite ongoing improvements, many patients do not experience clinical benefits. The tumor microenvironment is an important limiting factor in immunotherapy that has not been addressed fully in ACT treatments. In this study, we report that upregualtion of the immunosuppressive receptor programmed cell death-1 (PD-1) expressed on transferred T cells at the tumor site, in a murine model of ACT, compared with its expression on transferred T cells present in the peripheral blood and spleen. As PD-1 can attenuate T-cell-mediated antitumor responses, we tested whether its blockade with an anti-PD-1 antibody could enhance the antitumor activity of ACT in this model. Cotreatment with both agents increased the number of transferred T cells at the tumor site and also enhanced tumor regressions, compared with treatments with either agent alone. While anti-PD-1 did not reduce the number of immunosuppressive regulatory T cells and myeloid-derived suppressor cells present in tumor-bearing mice, we found that it increased expression of IFN-γ and CXCL10 at the tumor site. Bone marrow-transplant experiments using IFN-γR-/- mice implicated IFN-γ as a crucial nexus for controlling PD-1-mediated tumor infiltration by T cells. Taken together, our results imply that blocking the PD-1 pathway can increase IFN-γ at the tumor site, thereby increasing chemokine-dependent trafficking of immune cells into malignant disease sites.
Figures




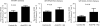
References
-
- Peddareddigari VR, Miller PW, Nebiyou BB, Overwijk WW, Ross MI, Lee JE, et al. Effect of clinical status and previous treatment of melanoma patients on expansion of TIL for adoptive T-cell therapy. ASCO Meeting Abstracts. 2009;27:3021.
-
- Laszlo G, Radvanyi CB, Minying Zhang, Priscilla Miller MG, Nicholas Papadopoulos, Patrick Hwu. Adoptive T Cell Therapy for Metastatic Melanoma: The MD Anderson Experience. Journal of Immunotherapy. 2010;33:863.
-
- Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

