Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration
- PMID: 22915768
- PMCID: PMC3477207
- DOI: 10.1124/jpet.112.195446
Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration
Abstract
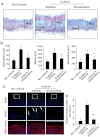
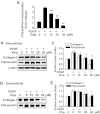
Abnormal vascular smooth muscle cell (SMC) activation is associated with various vascular disorders such as atherosclerosis, in-stent restenosis, vein graft disease, and transplantation-associated vasculopathy. Vinpocetine, a derivative of the alkaloid vincamine, has long been used as a cerebral blood flow enhancer for treating cognitive impairment. However, its role in pathological vascular remodeling remains unexplored. Herein, we show that systemic administration of vinpocetine significantly reduced neointimal formation in carotid arteries after ligation injury. Vinpocetine also markedly decreased spontaneous remodeling of human saphenous vein explants in ex vivo culture. In cultured SMCs, vinpocetine dose-dependently suppressed cell proliferation and caused G1-phase cell cycle arrest, which is associated with a decrease in cyclin D1 and an increase in p27Kip1 levels. In addition, vinpocetine dose-dependently inhibited platelet-derived growth factor (PDGF)-stimulated SMC migration as determined by the two-dimensional migration assays and three-dimensional aortic medial explant invasive assay. Moreover, vinpocetine significantly reduced PDGF-induced type I collagen and fibronectin expression. It is noteworthy that PDGF-stimulated phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2), but not protein kinase B, was specifically inhibited by vinpocetine. Vinpocetine powerfully attenuated intracellular reactive oxidative species (ROS) production, which largely mediates the inhibitory effects of vinpocetine on ERK1/2 activation and SMC growth. Taken together, our results reveal a novel function of vinpocetine in attenuating neointimal hyperplasia and pathological vascular remodeling, at least partially through suppressing ROS production and ERK1/2 activation in SMCs. Given the safety profile of vinpocetine, this study provides insight into the therapeutic potential of vinpocetine in proliferative vascular disorders.
Figures




References
-
- Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. (2009) Collagens in the progression and complications of atherosclerosis. Vasc Med 14:73–89 - PubMed
-
- Bagoly E, Fehér G, Szapáry L. (2007) [The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies]. Orv Hetil 148:1353–1358 - PubMed
-
- Balestreri R, Fontana L, Astengo F. (1987) A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc 35:425–430 - PubMed
-
- Bhardwaj S, Roy H, Ylä-Herttuala S. (2008) Gene therapy to prevent occlusion of venous bypass grafts. Expert Rev Cardiovasc Ther 6:641–652 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

