Isolation of novel synthetic prion strains by amplification in transgenic mice coexpressing wild-type and anchorless prion proteins
- PMID: 22915801
- PMCID: PMC3486332
- DOI: 10.1128/JVI.01353-12
Isolation of novel synthetic prion strains by amplification in transgenic mice coexpressing wild-type and anchorless prion proteins
Abstract
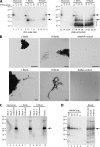
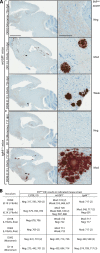
Mammalian prions are thought to consist of misfolded aggregates (protease-resistant isoform of the prion protein [PrP(res)]) of the cellular prion protein (PrP(C)). Transmissible spongiform encephalopathy (TSE) can be induced in animals inoculated with recombinant PrP (rPrP) amyloid fibrils lacking mammalian posttranslational modifications, but this induction is inefficient in hamsters or transgenic mice overexpressing glycosylphosphatidylinositol (GPI)-anchored PrP(C). Here we show that TSE can be initiated by inoculation of misfolded rPrP into mice that express wild-type (wt) levels of PrP(C) and that synthetic prion strain propagation and selection can be affected by GPI anchoring of the host's PrP(C). To create prions de novo, we fibrillized mouse rPrP in the absence of molecular cofactors, generating fibrils with a PrP(res)-like protease-resistant banding profile. These fibrils induced the formation of PrP(res) deposits in transgenic mice coexpressing wt and GPI-anchorless PrP(C) (wt/GPI(-)) at a combined level comparable to that of PrP(C) expression in wt mice. Secondary passage into mice expressing wt, GPI(-), or wt plus GPI(-) PrP(C) induced TSE disease with novel clinical, histopathological, and biochemical phenotypes. Contrary to laboratory-adapted mouse scrapie strains, the synthetic prion agents exhibited a preference for conversion of GPI(-) PrP(C) and, in one case, caused disease only in GPI(-) mice. Our data show that novel TSE agents can be generated de novo solely from purified mouse rPrP after amplification in mice coexpressing normal levels of wt and anchorless PrP(C). These observations provide insight into the minimal elements required to create prions in vitro and suggest that the PrP(C) GPI anchor can modulate the propagation of synthetic TSE strains.
Figures

Similar articles
-
Increased infectivity of anchorless mouse scrapie prions in transgenic mice overexpressing human prion protein.J Virol. 2015 Jun;89(11):6022-32. doi: 10.1128/JVI.00362-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810548 Free PMC article.
-
Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC.PLoS One. 2012;7(11):e48969. doi: 10.1371/journal.pone.0048969. Epub 2012 Nov 5. PLoS One. 2012. PMID: 23139828 Free PMC article.
-
PrP Knockout Cells Expressing Transmembrane PrP Resist Prion Infection.J Virol. 2017 Jan 3;91(2):e01686-16. doi: 10.1128/JVI.01686-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847358 Free PMC article.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
-
PrP P102L and Nearby Lysine Mutations Promote Spontaneous In Vitro Formation of Transmissible Prions.J Virol. 2017 Oct 13;91(21):e01276-17. doi: 10.1128/JVI.01276-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835493 Free PMC article.
-
A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers.PLoS Pathog. 2015 Jun 30;11(6):e1005017. doi: 10.1371/journal.ppat.1005017. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26125623 Free PMC article.
-
Increased infectivity of anchorless mouse scrapie prions in transgenic mice overexpressing human prion protein.J Virol. 2015 Jun;89(11):6022-32. doi: 10.1128/JVI.00362-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810548 Free PMC article.
-
Structural conservation of prion strain specificities in recombinant prion protein fibrils in real-time quaking-induced conversion.Prion. 2015;9(4):237-43. doi: 10.1080/19336896.2015.1062201. Prion. 2015. PMID: 26284507 Free PMC article.
-
Amyloid fibrils from the N-terminal prion protein fragment are infectious.Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):13851-13856. doi: 10.1073/pnas.1610716113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849581 Free PMC article.
References
-
- Atarashi R, et al. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 4:645–650 - PubMed
-
- Ayers JI, et al. 2011. The strain-encoded relationship between PrP replication, stability and processing in neurons is predictive of the incubation period of disease. PLoS Pathog. 7:e1001317 doi:10.1371/journal.ppat.1001317 - DOI - PMC - PubMed
-
- Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C. 2009. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog. 5:e1000421 doi:10.1371/journal.ppat.1000421 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

