Inclusion bodies are a site of ebolavirus replication
- PMID: 22915810
- PMCID: PMC3486333
- DOI: 10.1128/JVI.01525-12
Inclusion bodies are a site of ebolavirus replication
Abstract
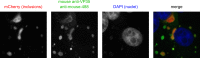
Inclusion bodies are a characteristic feature of ebolavirus infections in cells. They contain large numbers of preformed nucleocapsids, but their biological significance has been debated, and they have been suggested to be aggregates of viral proteins without any further biological function. However, recent data for other viruses that produce similar structures have suggested that inclusion bodies might be involved in genome replication and transcription. In order to study filovirus inclusion bodies, we fused mCherry to the ebolavirus polymerase L, which is found in inclusion bodies. The resulting L-mCherry fusion protein was functional in minigenome assays and incorporated into virus-like particles. Importantly, L-mCherry fluorescence in transfected cells was readily detectable and distributed in a punctate pattern characteristic for inclusion bodies. A recombinant ebolavirus encoding L-mCherry instead of L was rescued and showed virtually identical growth kinetics and endpoint titers to those for wild-type virus. Using this virus, we showed that the onset of inclusion body formation corresponds to the onset of viral genome replication, but that viral transcription occurs prior to inclusion body formation. Live-cell imaging further showed that inclusion bodies are highly dynamic structures and that they can undergo dramatic reorganization during cell division. Finally, by labeling nascent RNAs using click technology we showed that inclusion bodies are indeed the site of viral RNA synthesis. Based on these data we conclude that, rather than being inert aggregates of nucleocapsids, ebolavirus inclusion bodies are in fact complex and dynamic structures and an important site at which viral RNA replication takes place.
Figures





References
-
- Abramoff MD, Magalhaes PJ. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42
-
- Baskerville A, Fisher-Hoch SP, Neild GH, Dowsett AB. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199–209 - PubMed
-
- Beniac DR, et al. 2012. The organisation of Ebola virus reveals a capacity for extensive, modular polyploidy. PLoS One 7:e29608 doi:10.1371/journal.pone.0029608 - DOI - PMC - PubMed
-
- Boehmann Y, Enterlein S, Randolf A, Muhlberger E. 2005. A reconstituted replication and transcription system for Ebola virus Reston and comparison with Ebola virus Zaire. Virology 332:406–417 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

