Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a PSMA-dependent manner
- PMID: 22915856
- PMCID: PMC3419513
- DOI: 10.2147/IJN.S34381
Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a PSMA-dependent manner
Abstract
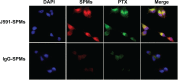
Background and methods: Problems with the clinical management of prostate cancer include the lack of both specific detection and efficient therapeutic intervention. We report the encapsulation of superparamagnetic iron platinum nanoparticles (SIPPs) and paclitaxel in a mixture of polyethyleneglycolated, fluorescent, and biotin-functionalized phospholipids to create multifunctional SIPP-PTX micelles (SPMs) that were conjugated to an antibody against prostate-specific membrane antigen (PSMA) for the specific targeting, magnetic resonance imaging (MRI), and treatment of human prostate cancer xenografts in mice.
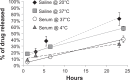
Results: SPMs were 45.4 ± 24.9 nm in diameter and composed of 160.7 ± 22.9 μg/mL iron, 247.0 ± 33.4 μg/mL platinum, and 702.6 ± 206.0 μg/mL paclitaxel. Drug release measurements showed that, at 37°C, half of the paclitaxel was released in 30.2 hours in serum and two times faster in saline. Binding assays suggested that PSMA-targeted SPMs specifically bound to C4-2 human prostate cancer cells in vitro and released paclitaxel into the cells. In vitro, paclitaxel was 2.2 and 1.6 times more cytotoxic than SPMs to C4-2 cells at 24 and 48 hours of incubation, respectively. After 72 hours of incubation, paclitaxel and SPMs were equally cytotoxic. SPMs had MRI transverse relaxivities of 389 ± 15.5 Hz/mM iron, and SIPP micelles with and without drug caused MRI contrast enhancement in vivo.
Conclusion: Only PSMA-targeted SPMs and paclitaxel significantly prevented growth of C4-2 prostate cancer xenografts in nude mice. Furthermore, mice injected with PSMA-targeted SPMs showed significantly more paclitaxel and platinum in tumors, compared with nontargeted SPM-injected and paclitaxel-injected mice.
Keywords: MRI; iron platinum; micelle; paclitaxel; prostate cancer.
Figures





Similar articles
-
Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen.Clin Cancer Res. 2006 Apr 15;12(8):2591-6. doi: 10.1158/1078-0432.CCR-05-2107. Clin Cancer Res. 2006. PMID: 16638870
-
PSMA Antibody-Conjugated Pentablock Copolymer Nanomicellar Formulation for Targeted Delivery to Prostate Cancer.AAPS PharmSciTech. 2018 Nov;19(8):3534-3549. doi: 10.1208/s12249-018-1126-9. Epub 2018 Aug 27. AAPS PharmSciTech. 2018. PMID: 30151731
-
Multifunctional iron platinum stealth immunomicelles: targeted detection of human prostate cancer cells using both fluorescence and magnetic resonance imaging.J Nanopart Res. 2011 Oct 1;13(10):4717-4729. doi: 10.1007/s11051-011-0439-3. J Nanopart Res. 2011. PMID: 22121333 Free PMC article.
-
Antibody-drug conjugates targeting prostate-specific membrane antigen.Front Biosci (Landmark Ed). 2014 Jan 1;19(1):12-33. doi: 10.2741/4193. Front Biosci (Landmark Ed). 2014. PMID: 24389170 Review.
-
Targeted Radionuclide Therapy of Prostate Cancer-From Basic Research to Clinical Perspectives.Molecules. 2020 Apr 10;25(7):1743. doi: 10.3390/molecules25071743. Molecules. 2020. PMID: 32290196 Free PMC article. Review.
Cited by
-
Longitudinal monitoring of microglial/macrophage activation in ischemic rat brain using Iba-1-specific nanoparticle-enhanced magnetic resonance imaging.J Cereb Blood Flow Metab. 2020 Dec;40(1_suppl):S117-S133. doi: 10.1177/0271678X20953913. Epub 2020 Sep 22. J Cereb Blood Flow Metab. 2020. PMID: 32960690 Free PMC article.
-
Development and In Vitro Evaluation of Liposomes Using Soy Lecithin to Encapsulate Paclitaxel.Int J Biomater. 2017;2017:8234712. doi: 10.1155/2017/8234712. Epub 2017 Feb 26. Int J Biomater. 2017. PMID: 28331495 Free PMC article.
-
Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer.Int J Mol Sci. 2017 May 20;18(5):1102. doi: 10.3390/ijms18051102. Int J Mol Sci. 2017. PMID: 28531102 Free PMC article. Review.
-
Intertumoral and intratumoral barriers as approaches for drug delivery and theranostics to solid tumors using stimuli-responsive materials.Mikrochim Acta. 2024 Aug 16;191(9):541. doi: 10.1007/s00604-024-06583-y. Mikrochim Acta. 2024. PMID: 39150483 Review.
-
Multifunctional polymeric micelles for delivery of drugs and siRNA.Front Pharmacol. 2014 Apr 25;5:77. doi: 10.3389/fphar.2014.00077. eCollection 2014. Front Pharmacol. 2014. PMID: 24795633 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Moul JW, Dawson N. Quality of life associated with treatment of castration-resistant prostate cancer: a review of the literature. Cancer Invest. 2012;30(1):1–12. - PubMed
-
- Beltran H, Beer TM, Carducci MA, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60(2):279–290. - PubMed
-
- Gomella LG, Gelpi F, Kelly WK. New treatment options for castrate-resistant prostate cancer: a urology perspective. Can J Urol. 2011;18(4):5767–5777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

