Development of Respimat(®) Soft Mist™ Inhaler and its clinical utility in respiratory disorders
- PMID: 22915941
- PMCID: PMC3417885
- DOI: 10.2147/MDER.S7409
Development of Respimat(®) Soft Mist™ Inhaler and its clinical utility in respiratory disorders
Abstract
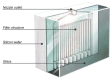
The Respimat(®) Soft Mist™ Inhaler (SMI) (Boehringer Ingelheim International GmbH, Ingelheim, Germany) was developed in response to the need for a pocket-sized device that can generate a single-breath, inhalable aerosol from a drug solution using a patient-independent, reproducible, and environmentally friendly energy supply. This paper describes the design and evolution of this innovative device from a laboratory concept model and the challenges that were overcome during its development and scaleup to mass production. A key technical breakthrough was the uniblock, a component combining filters and nozzles and made of silicon and glass, through which drug solution is forced using mechanical power. This allows two converging jets of solution to collide at a controlled angle, generating a fine aerosol of inhalable droplets. The mechanical energy comes from a spring which is tensioned by twisting the base of the device before use. Additional features of the Respimat(®) SMI include a dose indicator and a lockout mechanism to avoid the problems of tailing-off of dose size seen with pressurized metered dose inhalers. The Respimat(®) SMI aerosol cloud has a unique range of technical properties. The high fine particle fraction allied with the low velocity and long generation time of the aerosol translate into a higher fraction of the emitted dose being deposited in the lungs compared with aerosols from pressurized metered dose inhalers and dry powder inhalers. These advantages are realized in clinical trials in adults and children with obstructive lung diseases, which have shown that the efficacy and safety of a pressurized metered dose inhaler formulation of a combination bronchodilator can be matched by a Respimat(®) SMI formulation containing only one half or one quarter of the dose delivered by a pressurized metered dose inhaler. Patient satisfaction with the Respimat(®) SMI is high, and the long duration of the spray is of potential benefit to patients who have difficulty in coordinating inhalation with drug release.
Keywords: Respimat®; aerosol; deposition; drug delivery; inhaler device.
Figures















References
-
- Smith G, Hiller C, Mazumder R, Bone R. Aerodynamic size distribution of cromolyn sodium at ambient and airway humidity. Am Rev Respir Dis. 1980;121:513–517. - PubMed
-
- Köhler D, Fleischer W, Matthys H. New method for easy labeling of beta-2-agonists in the metered dose inhaler with technetium 99 m. Respiration. 1988;53:65–73. - PubMed
-
- Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3–21. - PubMed
-
- Crompton EK. Problems patients have using pressurized aerosol inhalers. Eur J Respir Dis Suppl. 1982;119:101–104. - PubMed
-
- Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191–194. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

