Development of a questionnaire to assess experience and preference of intranasal corticosteroids in patients with allergic rhinitis
- PMID: 22915972
- PMCID: PMC3417927
- DOI: 10.2147/PROM.S19195
Development of a questionnaire to assess experience and preference of intranasal corticosteroids in patients with allergic rhinitis
Abstract
Background: Allergic rhinitis affects 10%-20% of the US population. Its chronic nature, combined with patients' perceptions of safety/efficacy, administration, and sensory attributes of nasal sprays (corticosteroids), impact patient adherence to therapy. The purpose of this study was to develop a measure of experience with and preference for corticosteroid therapy for treatment of allergic rhinitis.
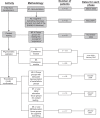
Methods: Questionnaire development was conducted through qualitative research including concept elicitation and content testing in 153 patients with allergic rhinitis. Patient focus groups (n = 66), in conjunction with content confirmation and saturation in additional groups (n = 87), provided research data. A literature-based conceptual framework was incorporated into the interview guide. An iterative process of data collection, analysis, and theory development yielded the conceptual framework.
Results: Consistent comments from the focus groups combined with those from cognitive debriefing interviews led to the incorporation of 14 finalized attributes into the Experience with Allergic Rhinitis Nasal Spray Questionnaire (EARNS-Q) items. Between the first and second cognitive debriefing interviews, researchers revised the EARNS-Q for retesting. Face and content validity tests indicated that the items, responses, and instructions were understood by study participants. The EARNS-Q is comprised of two modules that measure patient experience with nasal sprays (experience module), and patient preference for a nasal spray relative to another (preference module).
Conclusion: The EARNS-Q accurately measured patient experience with and preference for nasal sprays used in treating allergic rhinitis. A potential application of this questionnaire may be as a patient-reported outcomes endpoint in clinical trials of intranasal corticosteroids in patients with allergic rhinitis.
Keywords: EARNS-Q; allergic rhinitis; experience; intranasal corticosteroid; patient preference; questionnaire development.
Figures


Similar articles
-
Psychometric validation of the experience with allergic rhinitis nasal spray questionnaire.Patient Relat Outcome Meas. 2011 Jul;2:127-33. doi: 10.2147/PROM.S19371. Epub 2011 Jun 16. Patient Relat Outcome Meas. 2011. PMID: 22915973 Free PMC article.
-
Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids.Auris Nasus Larynx. 2013 Jun;40(3):277-81. doi: 10.1016/j.anl.2012.09.004. Epub 2012 Nov 3. Auris Nasus Larynx. 2013. PMID: 23127728 Clinical Trial.
-
Double-blind trials of azelastine nasal spray monotherapy versus combination therapy with loratadine tablets and beclomethasone nasal spray in patients with seasonal allergic rhinitis. Rhinitis Study Groups.Ann Allergy Asthma Immunol. 1999 Jun;82(6):535-41. doi: 10.1016/s1081-1206(10)63161-4. Ann Allergy Asthma Immunol. 1999. PMID: 10400480 Clinical Trial.
-
Evaluation of Intranasal Corticosteroid Sensory Attributes and Patient Preference for Fluticasone Furoate for the Treatment of Allergic Rhinitis.Clin Ther. 2019 Aug;41(8):1589-1596. doi: 10.1016/j.clinthera.2019.05.017. Epub 2019 Aug 8. Clin Ther. 2019. PMID: 31402060 Review.
-
Formulation considerations of intranasal corticosteroids for the treatment of allergic rhinitis.Ann Allergy Asthma Immunol. 2007 Jan;98(1):12-21. doi: 10.1016/S1081-1206(10)60854-X. Ann Allergy Asthma Immunol. 2007. PMID: 17225715 Review.
Cited by
-
Psychometric validation of the experience with allergic rhinitis nasal spray questionnaire.Patient Relat Outcome Meas. 2011 Jul;2:127-33. doi: 10.2147/PROM.S19371. Epub 2011 Jun 16. Patient Relat Outcome Meas. 2011. PMID: 22915973 Free PMC article.
References
-
- Mygind N, Dahl R. Epidemiology of allergic rhinitis. Pediatr Allergy Immunol. 1996;7:57–62. - PubMed
-
- Sibbald B. Epidemiology of allergic rhinitis. Monogr Allergy. 1993;31:61–79. - PubMed
-
- Wuthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int Arch Allergy Immunol. 1995;106:149–156. - PubMed
-
- Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: Complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1998;81:478–518. - PubMed
LinkOut - more resources
Full Text Sources

