Rates of gyrase supercoiling and transcription elongation control supercoil density in a bacterial chromosome
- PMID: 22916023
- PMCID: PMC3420936
- DOI: 10.1371/journal.pgen.1002845
Rates of gyrase supercoiling and transcription elongation control supercoil density in a bacterial chromosome
Abstract
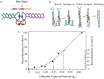
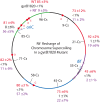
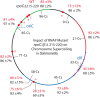
Gyrase catalyzes negative supercoiling of DNA in an ATP-dependent reaction that helps condense bacterial chromosomes into a compact interwound "nucleoid." The supercoil density (σ) of prokaryotic DNA occurs in two forms. Diffusible supercoil density (σ(D)) moves freely around the chromosome in 10 kb domains, and constrained supercoil density (σ(C)) results from binding abundant proteins that bend, loop, or unwind DNA at many sites. Diffusible and constrained supercoils contribute roughly equally to the total in vivo negative supercoil density of WT cells, so σ = σ(C)+σ(D). Unexpectedly, Escherichia coli chromosomes have a 15% higher level of σ compared to Salmonella enterica. To decipher critical mechanisms that can change diffusible supercoil density of chromosomes, we analyzed strains of Salmonella using a 9 kb "supercoil sensor" inserted at ten positions around the genome. The sensor contains a complete Lac operon flanked by directly repeated resolvase binding sites, and the sensor can monitor both supercoil density and transcription elongation rates in WT and mutant strains. RNA transcription caused (-) supercoiling to increase upstream and decrease downstream of highly expressed genes. Excess upstream supercoiling was relaxed by Topo I, and gyrase replenished downstream supercoil losses to maintain an equilibrium state. Strains with TS gyrase mutations growing at permissive temperature exhibited significant supercoil losses varying from 30% of WT levels to a total loss of σ(D) at most chromosome locations. Supercoil losses were influenced by transcription because addition of rifampicin (Rif) caused supercoil density to rebound throughout the chromosome. Gyrase mutants that caused dramatic supercoil losses also reduced the transcription elongation rates throughout the genome. The observed link between RNA polymerase elongation speed and gyrase turnover suggests that bacteria with fast growth rates may generate higher supercoil densities than slow growing species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Roca J (1995) The mechanisms of DNA topoisomerases. Trends Biochem Sci 20: 156–160. - PubMed
-
- DiNardo S, Voelkel KA, Sternglanz R, Reynolds AE, Wright A (1982) Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell 31: 43–51. - PubMed
-
- Drolet M, Broccoli S, Rallu F, Hraiky C, Fortin C, et al. (2003) The problem of hypernegative supercoiling and R-loop formation in transcription. Front Biosci 8: D210–D221. - PubMed
-
- DiGate RJ, Marians KJ (1988) Identification of a potent decatenating enzyme from Escherichia coli. J Biol Chem 263: 13366–13373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

