Transcriptional programs controlling perinatal lung maturation
- PMID: 22916088
- PMCID: PMC3423373
- DOI: 10.1371/journal.pone.0037046
Transcriptional programs controlling perinatal lung maturation
Abstract
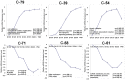
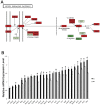
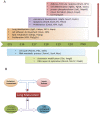
The timing of lung maturation is controlled precisely by complex genetic and cellular programs. Lung immaturity following preterm birth frequently results in Respiratory Distress Syndrome (RDS) and Broncho-Pulmonary Dysplasia (BPD), which are leading causes of mortality and morbidity in preterm infants. Mechanisms synchronizing gestational length and lung maturation remain to be elucidated. In this study, we designed a genome-wide mRNA expression time-course study from E15.5 to Postnatal Day 0 (PN0) using lung RNAs from C57BL/6J (B6) and A/J mice that differ in gestational length by ∼30 hr (B6<A/J). Comprehensive bioinformatics and functional genomics analyses were used to identify key regulators, bioprocesses and transcriptional networks controlling lung maturation. We identified both temporal and strain dependent gene expression patterns during lung maturation. For time dependent changes, cell adhesion, vasculature development, and lipid metabolism/transport were major bioprocesses induced during the saccular stage of lung development at E16.5-E17.5. CEBPA, PPARG, VEGFA, CAV1 and CDH1 were found to be key signaling and transcriptional regulators of these processes. Innate defense/immune responses were induced at later gestational ages (E18.5-20.5), STAT1, AP1, and EGFR being important regulators of these responses. Expression of RNAs associated with the cell cycle and chromatin assembly was repressed during prenatal lung maturation and was regulated by FOXM1, PLK1, chromobox, and high mobility group families of transcription factors. Strain dependent lung mRNA expression differences peaked at E18.5. At this time, mRNAs regulating surfactant and innate immunity were more abundantly expressed in lungs of B6 (short gestation) than in A/J (long gestation) mice, while expression of genes involved in chromatin assembly and histone modification were expressed at lower levels in B6 than in A/J mice. The present study systemically mapped key regulators, bioprocesses, and transcriptional networks controlling lung maturation, providing the basis for new therapeutic strategies to enhance lung function in preterm infants.
Conflict of interest statement
Figures






References
-
- Goldenberg RL, Rouse DJ (1998) Prevention of premature birth. N Engl J Med 339: 313–320. - PubMed
-
- Dubin SB (1990) Assessment of fetal lung maturity: in search of the Holy Grail. Clin Chem 36: 1867–1869. - PubMed
-
- Grenache DG, Gronowski AM (2006) Fetal lung maturity. Clin Biochem 39: 1–10. - PubMed
-
- McMurtry IF (2002) Introduction: pre- and postnatal lung development, maturation, and plasticity. Am J Physiol Lung Cell Mol Physiol 282: L341–344. - PubMed
-
- Whitsett JA, Matsuzaki Y (2006) Transcriptional regulation of perinatal lung maturation. Pediatr Clin North Am 53: viii–887, viii, 873-887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

