Role of the dopaminergic system in the acquisition, expression and reinstatement of MDMA-induced conditioned place preference in adolescent mice
- PMID: 22916213
- PMCID: PMC3420895
- DOI: 10.1371/journal.pone.0043107
Role of the dopaminergic system in the acquisition, expression and reinstatement of MDMA-induced conditioned place preference in adolescent mice
Abstract
Background: The rewarding effects of 3,4-methylenedioxy-metamphetamine (MDMA) have been demonstrated in conditioned place preference (CPP) procedures, but the involvement of the dopaminergic system in MDMA-induced CPP and reinstatement is poorly understood.
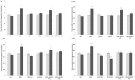
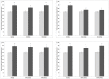
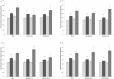
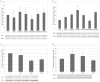
Methodology/principal findings: In this study, the effects of the DA D1 antagonist SCH 23390 (0.125 and 0.250 mg/kg), the DA D2 antagonist Haloperidol (0.1 and 0.2 mg/kg), the D2 antagonist Raclopride (0.3 and 0.6 mg/kg) and the dopamine release inhibitor CGS 10746B (3 and 10 mg/kg) on the acquisition, expression and reinstatement of a CPP induced by 10 mg/kg of MDMA were evaluated in adolescent mice. As expected, MDMA significantly increased the time spent in the drug-paired compartment during the post-conditioning (Post-C) test, and a priming dose of 5 mg/kg reinstated the extinguished preference. The higher doses of Haloperidol, Raclopride and CGS 10746B and both doses of SCH 23390 blocked acquisition of the MDMA-induced CPP. However, only Haloperidol blocked expression of the CPP. Reinstatement of the extinguished preference was not affected by any of the drugs studied. Analysis of brain monoamines revealed that the blockade of CPP acquisition was accompanied by an increase in DA concentration in the striatum, with a concomitant decrease in DOPAC and HVA levels. Administration of haloperidol during the Post-C test produced increases in striatal serotonin, DOPAC and HVA concentrations. In mice treated with the higher doses of haloperidol and CGS an increase in SERT concentration in the striatum was detected during acquisition of the CPP, but no changes in DAT were observed.
Conclusions/significance: These results demonstrate that, in adolescent mice, the dopaminergic system is involved in the acquisition and expression of MDMA-induced CPP, but not in its reinstatement.
Conflict of interest statement
Figures
Similar articles
-
Rewarding effects and reinstatement of MDMA-induced CPP in adolescent mice.Neuropsychopharmacology. 2007 Aug;32(8):1750-9. doi: 10.1038/sj.npp.1301309. Epub 2007 Feb 14. Neuropsychopharmacology. 2007. PMID: 17299518
-
Assessment of the abuse potential of MDMA in the conditioned place preference paradigm: role of CB1 receptors.Prog Neuropsychopharmacol Biol Psychiatry. 2013 Dec 2;47:77-84. doi: 10.1016/j.pnpbp.2013.07.013. Epub 2013 Aug 17. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23959085
-
Involvement of 5-hydroxytryptamine 5-HT₃ serotonergic receptors in the acquisition and reinstatement of the conditioned place preference induced by MDMA.Eur J Pharmacol. 2013 Aug 15;714(1-3):132-41. doi: 10.1016/j.ejphar.2013.06.005. Epub 2013 Jun 20. Eur J Pharmacol. 2013. PMID: 23792143
-
Effects of risperidone on the acquisition and reinstatement of the conditioned place preference induced by MDMA.Brain Res Bull. 2013 Sep;98:36-43. doi: 10.1016/j.brainresbull.2013.07.009. Epub 2013 Jul 24. Brain Res Bull. 2013. PMID: 23892054
-
CGS 10746B, a novel dopamine release inhibitor, blocks the establishment of cocaine and MDMA conditioned place preferences.Pharmacol Biochem Behav. 1998 Jan;59(1):215-20. doi: 10.1016/s0091-3057(97)00424-3. Pharmacol Biochem Behav. 1998. PMID: 9443558
Cited by
-
Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine.ACS Chem Neurosci. 2018 Oct 17;9(10):2408-2427. doi: 10.1021/acschemneuro.8b00155. Epub 2018 Jul 12. ACS Chem Neurosci. 2018. PMID: 30001118 Free PMC article. Review.
-
The Comparison Between Positive and Negative Symptoms Severity in Prolonged Methamphetamine-induced Psychotic Disorder and Schizophrenia.Basic Clin Neurosci. 2022 May-Jun;13(3):325-333. doi: 10.32598/bcn.2021.2837.1. Epub 2022 May 1. Basic Clin Neurosci. 2022. PMID: 36457876 Free PMC article.
-
5-HT2C receptors in the nucleus accumbens constrain the rewarding effects of MDMA.Mol Psychiatry. 2025 Jul 24. doi: 10.1038/s41380-025-03128-4. Online ahead of print. Mol Psychiatry. 2025. PMID: 40707786
-
Dose and time-dependent selective neurotoxicity induced by mephedrone in mice.PLoS One. 2014 Jun 3;9(6):e99002. doi: 10.1371/journal.pone.0099002. eCollection 2014. PLoS One. 2014. PMID: 24892744 Free PMC article.
-
Systemic enhancement of serotonin signaling reverses social deficits in multiple mouse models for ASD.Neuropsychopharmacology. 2021 Oct;46(11):2000-2010. doi: 10.1038/s41386-021-01091-6. Epub 2021 Jul 8. Neuropsychopharmacology. 2021. PMID: 34239048 Free PMC article.
References
-
- Solowij N, Hall W, Lee N (1992) Recreational MDMA use in Sydney: a profile of ecstasy users and their experiences with the drug. Br J Addict 87: 1161–1172. - PubMed
-
- George J, Kinner SA, Bruno R, Degenhardt L, Dunn M (2010) Contextualising psychological distress among regular ecstasy users: the importance of sociodemographic factors and patterns of drug use. Drug Alcohol Rev 29: 243–249. - PubMed
-
- Leung KS, Cottler LB (2008) Ecstasy and other club drugs: a review of recent epidemiologic studies. Curr Opin Psychiatry 21: 234–241. - PubMed
-
- Robledo P, Mendizabal V, Ortuño J, de la Torre R, Kieffer BL, et al. (2004b) The rewarding properties of MDMA are preserved in mice lacking mu-opioid receptors. Eur J Neurosci 20: 853–858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

