Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice
- PMID: 22916251
- PMCID: PMC3420877
- DOI: 10.1371/journal.pone.0043376
Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice
Abstract
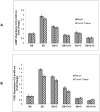
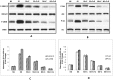
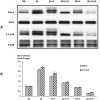
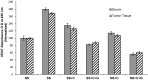
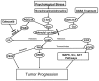
Pancreatic ductal adenocarcinoma (PDAC) has a poor prognosis and is associated with high levels of psychological distress. We have shown that beta-adrenergic receptors (β-ARs), which are activated by stress neurotransmitters, regulate PDAC cells via cyclic AMP (cAMP)-dependent signaling in vitro, that social stress promotes PDAC progression in mouse xenografts and that γ-aminobutyric acid (GABA) inhibits these responses in vitro and in vivo. The targeted inhibition of stress-induced regulatory pathways may abolish the potentially negative impact of psychological stress on clinical outcomes. Our current data show that chronic exposure of PDAC cell lines Panc-1 (activating point mutations in K-ras) and BXPC-3 (no mutations in K-ras) in vitro to the stress neurotransmitter epinephrine at the concentration (15 nM) previously measured in the serum of mice exposed to social stress significantly increased proliferation and migration. These responses were inhibited in a concentration-dependent manner by celecoxib. The effects of celecoxib alone and in combination with γ-aminobutyric acid (GABA) on the progression of subcutaneous mouse xenografts from the cell line (BXPC-3) most responsive to epinephrine were then investigated in the presence and absence of social stress. Cancer-stimulating factors (VEGF & prostaglandin E(2) [PGE(2)]) and levels of cAMP were measured by immunoassays in blood and xenograft tissue. Phosphorylation of the signaling proteins ERK, CREB, Src, and AKT was assessed by ELISA assays and Western blotting. Expression of COX-2, 5-lipoxygenase, and p-5-LOX were determined by semi-quantitative Western blotting. Celecoxib alone significantly inhibited xenograft progression and decreased systemic and tumor VEGF, PGE2, and cAMP as well as phosphorylated signaling proteins in stress-exposed and stress-free mice. These responses were significantly enhanced by co-treatment with GABA. The celecoxib-induced downregulation of COX-2 protein and p-5-LOX was also significantly enhanced by GABA under both experimental conditions. Our findings identify the targeted inhibition of stress-induced pathways as a promising area for more effective cancer intervention in pancreatic cancer.
Conflict of interest statement
Figures
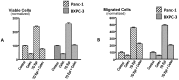


References
-
- World Health Organization (2012) Cancer fact sheet. Available: http://www.who.int/mediacenter/factsheets/fs297/en/index.htm. Accessed 2011 Mar 30.
-
- Almhanna K, Philip PA (2011) Defining new paradigms for the treatment of pancreatic cancer. Curr Treat Options Oncol 12: 111–125. - PubMed
-
- Nieto J, Grossbard ML, Kozuch P (2008) Metastatic pancreatic cancer 2008: Is the glass less empty? Oncologist 13: 562–576. - PubMed
-
- Schuller HM (2009) Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer 9: 195–205. - PubMed
-
- Melhem-Bertrandt A, Sood AK (2012) New directions in reducing stress effects on cancer. Cancer Prev Res 5: 147–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

