Vacuolar protein sorting receptor in Giardia lamblia
- PMID: 22916299
- PMCID: PMC3423367
- DOI: 10.1371/journal.pone.0043712
Vacuolar protein sorting receptor in Giardia lamblia
Abstract
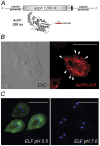
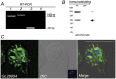
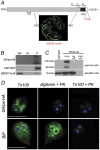
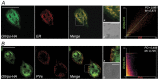
In Giardia, lysosome-like peripheral vacuoles (PVs) need to specifically coordinate their endosomal and lysosomal functions to be able to successfully perform endocytosis, protein degradation and protein delivery, but how cargo, ligands and molecular components generate specific routes to the PVs remains poorly understood. Recently, we found that delivering membrane Cathepsin C and the soluble acid phosphatase (AcPh) to the PVs is adaptin (AP1)-dependent. However, the receptor that links AcPh and AP1 was never described. We have studied protein-binding to AcPh by using H6-tagged AcPh, and found that a membrane protein interacted with AcPh. This protein, named GlVps (for Giardia lamblia Vacuolar protein sorting), mainly localized to the ER-nuclear envelope and in some PVs, probably functioning as the sorting receptor for AcPh. The tyrosine-binding motif found in the C-terminal cytoplasmic tail domain of GlVps was essential for its exit from the endoplasmic reticulum and transport to the vacuoles, with this motif being necessary for the interaction with the medium subunit of AP1. Thus, the mechanism by which soluble proteins, such as AcPh, reach the peripheral vacuoles in Giardia appears to be very similar to the mechanism of lysosomal protein-sorting in more evolved eukaryotic cells.
Conflict of interest statement
Figures

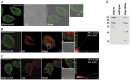
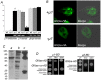
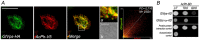

References
-
- Rodriguez-Boulan E, Musch A (2005) Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta 1744: 455–464. - PubMed
-
- Braulke T, Bonifacino JS (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793: 605–614. - PubMed
-
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77: 579–586. - PubMed
-
- Winther JR, Stevens TH, Kielland-Brandt MC (1991) Yeast carboxypeptidase Y requires glycosylation for efficient intracellular transport, but not for vacuolar sorting, in vivo stability, or activity. Eur J Biochem 197: 681–689. - PubMed
-
- Jorgensen MU, Emr SD, Winther JR (1999) Ligand recognition and domain structure of Vps10p, a vacuolar protein sorting receptor in Saccharomyces cerevisiae. Eur J Biochem 260: 461–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

