Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii
- PMID: 22916306
- PMCID: PMC3423376
- DOI: 10.1371/journal.pone.0043773
Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii
Abstract
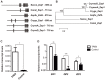
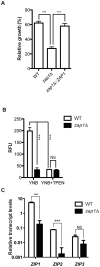
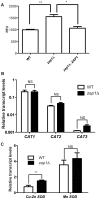
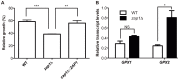
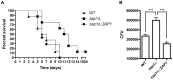
Zinc homeostasis is essential for fungal growth, as this metal is a critical structural component of several proteins, including transcription factors. The fungal pathogen Cryptococcus gattii obtains zinc from the stringent zinc-limiting milieu of the host during the infection process. To characterize the zinc metabolism in C. gattii and its relationship to fungal virulence, the zinc finger protein Zap1 was functionally characterized. The C. gattii ZAP1 gene is an ortholog of the master regulatory genes zafA and ZAP1 that are found in Aspergillus fumigatus and Saccharomyces cerevisiae, respectively. There is some evidence to support an association between Zap1 and zinc metabolism in C. gattii: (i) ZAP1 expression is highly induced during zinc deprivation, (ii) ZAP1 knockouts demonstrate impaired growth in zinc-limiting conditions, (iii) Zap1 regulates the expression of ZIP zinc transporters and distinct zinc-binding proteins and (iv) Zap1 regulates the labile pool of intracellular zinc. In addition, the deletion of ZAP1 reduces C. gattii virulence in a murine model of cryptococcosis infection. Based on these observations, we postulate that proper zinc metabolism plays a crucial role in cryptococcal virulence.
Conflict of interest statement
Figures

Similar articles
-
Effects of zinc transporters on Cryptococcus gattii virulence.Sci Rep. 2015 May 7;5:10104. doi: 10.1038/srep10104. Sci Rep. 2015. PMID: 25951314 Free PMC article.
-
Zrg1, a cryptococcal protein associated with regulation of growth in nutrient deprivation conditions.Genomics. 2021 Mar;113(2):805-814. doi: 10.1016/j.ygeno.2021.01.023. Epub 2021 Jan 30. Genomics. 2021. PMID: 33529779
-
Chitosan Biosynthesis and Virulence in the Human Fungal Pathogen Cryptococcus gattii.mSphere. 2019 Oct 9;4(5):e00644-19. doi: 10.1128/mSphere.00644-19. mSphere. 2019. PMID: 31597720 Free PMC article.
-
Fungal zinc metabolism and its connections to virulence.Front Cell Infect Microbiol. 2013 Oct 14;3:65. doi: 10.3389/fcimb.2013.00065. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24133658 Free PMC article. Review.
-
Etiological factors of cryptococcosis - what makes them pathogens ?Med Dosw Mikrobiol. 2015;67(3-4):221-31. Med Dosw Mikrobiol. 2015. PMID: 27019916 Review.
Cited by
-
Zinc Starvation Induces Cell Wall Remodeling and Activates the Antioxidant Defense System in Fonsecaea pedrosoi.J Fungi (Basel). 2024 Jan 31;10(2):118. doi: 10.3390/jof10020118. J Fungi (Basel). 2024. PMID: 38392790 Free PMC article.
-
The ZIP family zinc transporters support the virulence of Cryptococcus neoformans.Med Mycol. 2016 Aug 1;54(6):605-15. doi: 10.1093/mmy/myw013. Epub 2016 Apr 26. Med Mycol. 2016. PMID: 27118799 Free PMC article.
-
Vitamin Biosynthesis as an Antifungal Target.J Fungi (Basel). 2018 Jun 17;4(2):72. doi: 10.3390/jof4020072. J Fungi (Basel). 2018. PMID: 29914189 Free PMC article. Review.
-
sRNAs as possible regulators of retrotransposon activity in Cryptococcus gattii VGII.BMC Genomics. 2017 Apr 12;18(1):294. doi: 10.1186/s12864-017-3688-4. BMC Genomics. 2017. PMID: 28403818 Free PMC article.
-
ZafA Gene Is Important for Trichophyton mentagrophytes Growth and Pathogenicity.Int J Mol Sci. 2019 Feb 15;20(4):848. doi: 10.3390/ijms20040848. Int J Mol Sci. 2019. PMID: 30781401 Free PMC article.
References
-
- Ehrensberger KM, Bird AJ (2011) Hammering out details: regulating metal levels in eukaryotes. Trends Biochem Sci 36: 524–531. - PubMed
-
- Eide DJ (1998) The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr 18: 441–469. - PubMed
-
- Waters BM, Eide DJ (2002) Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J Biol Chem 277: 33749–33757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

