Phospholipid-polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery
- PMID: 22916337
- PMCID: PMC3423223
- DOI: 10.1007/s13346-010-0004-0
Phospholipid-polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery
Abstract
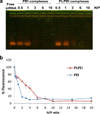
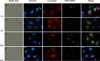
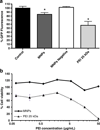
Gene silencing using small interfering RNA (siRNA) is a promising therapeutic strategy for the treatment of various diseases, in particular, cancer. Recently, our group reported on a novel gene carrier, the micelle-like nanoparticle (MNP), based on the combination of a covalent conjugate of phospholipid and polyethylenimine (PLPEI) with polyethylene glycol (PEG) and lipids. These long-circulating MNPs loaded with plasmid DNA-mediated gene expression in distal tumors after systemic administration in vivo. In the current study, we investigated the potential of MNPs for siRNA delivery. MNPs were prepared by condensing siRNA with PLPEI at a nitrogen/phosphate ratio of 10, where the binding of siRNA is complete. The addition of a PEG/lipid coating to the PLPEI complexes generated particles with sizes of ca. 200 nm and a neutral surface charge compared with positively charged PLPEI polyplexes without the additional coating. MNPs protected the loaded siRNA against enzymatic digestion and enhanced the cellular uptake of the siRNA payload. MNPs carrying green fluorescent protein (GFP)-targeted siRNA effectively downregulated the gene in cells that stably express GFP. Finally, MNPs were non-toxic at a wide range of concentrations and for different cell lines.
Figures




References
-
- Alshamsan A, Haddadi A, Incani V, Samuel J, Lavasanifar A, Uludag H. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol Pharmaceutics. 2009;6:121–133. - PubMed
-
- Chollet P, Favrot MC, Hurbin A, Coll JL. Side-effects of a systemic injection of linear polyethylenimine–DNA complexes. J Gene Med. 2002;4:84–91. - PubMed
-
- Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45:268–275. - PubMed
-
- Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:1868–1876. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

