A method for the production of D-tagatose using a recombinant Pichia pastoris strain secreting β-D-galactosidase from Arthrobacter chlorophenolicus and a recombinant L-arabinose isomerase from Arthrobacter sp. 22c
- PMID: 22917022
- PMCID: PMC3520711
- DOI: 10.1186/1475-2859-11-113
A method for the production of D-tagatose using a recombinant Pichia pastoris strain secreting β-D-galactosidase from Arthrobacter chlorophenolicus and a recombinant L-arabinose isomerase from Arthrobacter sp. 22c
Abstract
Background: D-Tagatose is a natural monosaccharide which can be used as a low-calorie sugar substitute in food, beverages and pharmaceutical products. It is also currently being tested as an anti-diabetic and obesity control drug. D-Tagatose is a rare sugar, but it can be manufactured by the chemical or enzymatic isomerization of D-galactose obtained by a β-D-galactosidase-catalyzed hydrolysis of milk sugar lactose and the separation of D-glucose and D-galactose. L-Arabinose isomerases catalyze in vitro the conversion of D-galactose to D-tagatose and are the most promising enzymes for the large-scale production of D-tagatose.
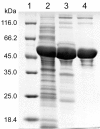
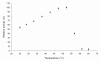
Results: In this study, the araA gene from psychrotolerant Antarctic bacterium Arthrobacter sp. 22c was isolated, cloned and expressed in Escherichia coli. The active form of recombinant Arthrobacter sp. 22c L-arabinose isomerase consists of six subunits with a combined molecular weight of approximately 335 kDa. The maximum activity of this enzyme towards D-galactose was determined as occurring at 52°C; however, it exhibited over 60% of maximum activity at 30°C. The recombinant Arthrobacter sp. 22c L-arabinose isomerase was optimally active at a broad pH range of 5 to 9. This enzyme is not dependent on divalent metal ions, since it was only marginally activated by Mg2+, Mn2+ or Ca2+ and slightly inhibited by Co2+ or Ni2+. The bioconversion yield of D-galactose to D-tagatose by the purified L-arabinose isomerase reached 30% after 36 h at 50°C. In this study, a recombinant Pichia pastoris yeast strain secreting β-D-galactosidase Arthrobacter chlorophenolicus was also constructed. During cultivation of this strain in a whey permeate, lactose was hydrolyzed and D-glucose was metabolized, whereas D-galactose was accumulated in the medium. Moreover, cultivation of the P. pastoris strain secreting β-D-galactosidase in a whey permeate supplemented with Arthrobacter sp. 22c L-arabinose isomerase resulted in a 90% yield of lactose hydrolysis, the complete utilization of D-glucose and a 30% conversion of D-galactose to D-tagatose.
Conclusions: The method developed for the simultaneous hydrolysis of lactose, utilization of D-glucose and isomerization of D-galactose using a P. pastoris strain secreting β-D-galactosidase and recombinant L-arabinose isomerase seems to offer an interesting alternative for the production of D-tagatose from lactose-containing feedstock.
Figures





Similar articles
-
Enzymatic conversion of D-galactose to D-tagatose: heterologous expression and characterisation of a thermostable L-arabinose isomerase from Thermoanaerobacter mathranii.Appl Microbiol Biotechnol. 2004 Jun;64(6):816-22. doi: 10.1007/s00253-004-1578-6. Epub 2004 Feb 19. Appl Microbiol Biotechnol. 2004. PMID: 15168095
-
Comparison of Two l-Arabinose Isomerases for Multienzymatic Conversion of Lactose in Skim Milk Permeate at Neutral and Acidic pH.J Agric Food Chem. 2025 Jun 25;73(25):15889-15899. doi: 10.1021/acs.jafc.5c04545. Epub 2025 Jun 12. J Agric Food Chem. 2025. PMID: 40504116 Free PMC article.
-
The acid-tolerant L-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures.Microb Cell Fact. 2011 Nov 10;10:96. doi: 10.1186/1475-2859-10-96. Microb Cell Fact. 2011. PMID: 22074172 Free PMC article.
-
Recent Advances on Biological Production of a Functional Low-Calorie Sugar d-Tagatose.J Agric Food Chem. 2025 Jul 30;73(30):18511-18524. doi: 10.1021/acs.jafc.5c04610. Epub 2025 Jul 21. J Agric Food Chem. 2025. PMID: 40690603 Review.
-
Current studies on biological tagatose production using L-arabinose isomerase: a review and future perspective.Appl Microbiol Biotechnol. 2004 Aug;65(3):243-9. doi: 10.1007/s00253-004-1665-8. Epub 2004 Jul 10. Appl Microbiol Biotechnol. 2004. PMID: 15248040 Review.
Cited by
-
Cloning, Expression, and Characterization of a Novel L-Arabinose Isomerase from the Psychrotolerant Bacterium Pseudoalteromonas haloplanktis.Mol Biotechnol. 2016 Nov;58(11):695-706. doi: 10.1007/s12033-016-9969-3. Mol Biotechnol. 2016. PMID: 27586234
-
Overcoming the thermodynamic equilibrium of an isomerization reaction through oxidoreductive reactions for biotransformation.Nat Commun. 2019 Mar 22;10(1):1356. doi: 10.1038/s41467-019-09288-6. Nat Commun. 2019. PMID: 30902987 Free PMC article.
-
Biosynthesis of rare hexoses using microorganisms and related enzymes.Beilstein J Org Chem. 2013 Nov 12;9:2434-45. doi: 10.3762/bjoc.9.281. Beilstein J Org Chem. 2013. PMID: 24367410 Free PMC article. Review.
-
A New Expression System Based on Psychrotolerant Debaryomyces macquariensis Yeast and Its Application to the Production of Cold-Active β-d-Galactosidase from Paracoccus sp. 32d.Int J Mol Sci. 2022 Oct 2;23(19):11691. doi: 10.3390/ijms231911691. Int J Mol Sci. 2022. PMID: 36232994 Free PMC article.
-
Pentose degradation in archaea: Halorhabdus species degrade D-xylose, L-arabinose and D-ribose via bacterial-type pathways.Extremophiles. 2020 Sep;24(5):759-772. doi: 10.1007/s00792-020-01192-y. Epub 2020 Aug 5. Extremophiles. 2020. PMID: 32761262 Free PMC article.
References
-
- Hirst EL, Hough L, Jones JKN. Composition of the gum of Sterculia setigera: occurrence of D-tagatose in nature. Nature. 1949;163:177. - PubMed
-
- Troyano E, Villamiel M, Olano A, Sanz J, Martinez-Castro I. Monosaccharides and myo-inositol in commercial milks. J Agric Food Chem. 1996;44:815–817. doi: 10.1021/jf950260d. - DOI
-
- Levin GV, Zehner LR, Saunders JP, Beadle JR. Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr. 1995;62:1161S–1168S. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

