The discovery of [Ni(NHC)RCN]2 species and their role as cycloaddition catalysts for the formation of pyridines
- PMID: 22917161
- PMCID: PMC3480329
- DOI: 10.1021/ja3075924
The discovery of [Ni(NHC)RCN]2 species and their role as cycloaddition catalysts for the formation of pyridines
Abstract
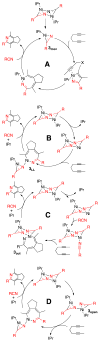
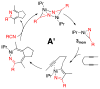
The reaction of Ni(COD)(2), IPr, and nitrile affords dimeric [Ni(IPr)RCN](2) in high yields. X-ray analysis revealed these species display simultaneous η(1)- and η(2)-nitrile binding modes. These dimers are catalytically competent in the formation of pyridines from the cycloaddition of diynes and nitriles. Kinetic analysis showed the reaction to be first order in [Ni(IPr)RCN](2), zeroth order in added IPr, zeroth order in nitrile, and zeroth order in diyne. Extensive stoichiometric competition studies were performed, and selective incorporation of the exogenous, not dimer bound, nitrile was observed. Post cycloaddition, the dimeric state was found to be largely preserved. Nitrile and ligand exchange experiments were performed and found to be inoperative in the catalytic cycle. These observations suggest a mechanism whereby the catalyst is activated by partial dimer-opening followed by binding of exogenous nitrile and subsequent oxidative heterocoupling.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
-
-
For leading sources see: Jones G. Pyridines and their benzoderivatives: synthesis. In: Katritzky A, Rees CW, Scriven EFV, editors. Comprehensive Heterocyclic Chemistry II. Vol. 5. Pergamon; Oxford: 1996. p. 167.Alford PE. Six-Membered Ring Systems: Pyridines and Their Benzo Derivatives. In: Gribble GW, Joule JA, editors. Progress in Heterocyclic Chemistry. Vol. 22. Elsevier; Oxford: 2011. p. 349.Gonzalez-Bello C, Castedo L. Six-membered Heterocycles: Pyridines. In. In: Alvarez-Builla J, Vaquero JJ, Barluenga J, editors. Modern Heterocyclic Chemistry. Vol. 3. Wiley-VHC; Weinheim: 2011. p. 1431.
-
-
- Weng CM, Hong FE. Organometallics. 2011;30:3740.
- Dahy AA, Koga N. J Organomet Chem. 2010;695:2240.
- Kase K, Goswami A, Ohtaki K, Tanabe E, Saino N, Okamoto S. Org Lett. 2007;9:931. - PubMed
- Wakatsuki Y, Yamazaki H. J Chem Soc Chem Comm. 1973;8:280.
- Wakatsuki Y, Yamazaki H. J Chem Soc Dalton. 1978;10:1278.
- Bonnemann H, Brinkmann R, Schenkluhn H. Synthesis. 1974;8:575.
- Naiman A, Vollhardt KPC. Angew Chem. 1977;89:758.
-
- Yamamoto Y, Kinpara K, Ogawa R, Nishiyama H, Itoh K. Chem Eur J. 2006;12:5618. - PubMed
- Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K. J Am Chem Soc. 2005;127:605. - PubMed
- Yamamoto Y, Kinpara K, Nishiyama H, Itoh K. Adv Synth Catal. 2005;347:1913.
- Yamamoto Y, Ogawa R, Itoh K. J Am Chem Soc. 2001;123:6189. - PubMed
- Yamamoto Y, Okuda S, Itoh K. Chem Commun. 2001:1102.
- Varela JA, Castedo L, Saa C. J Org Chem. 2003;68:8595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

