Changes in response properties of rostral ventromedial medulla neurons during prolonged inflammation: modulation by neurokinin-1 receptors
- PMID: 22917610
- PMCID: PMC3498481
- DOI: 10.1016/j.neuroscience.2012.08.029
Changes in response properties of rostral ventromedial medulla neurons during prolonged inflammation: modulation by neurokinin-1 receptors
Abstract
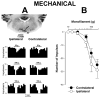
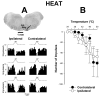
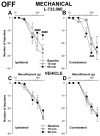
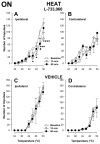
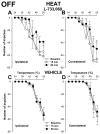
Activation of neurokinin-1 (NK-1) receptors in the rostral ventromedial medulla (RVM) can facilitate pain transmission in conditions such as inflammation, and thereby contribute to hyperalgesia. Since blockade of NK-1 receptors in the RVM can attenuate hyperalgesia produced by prolonged inflammation, we examined the role of NK-1 receptors in changes of response properties of RVM neurons following four days of hind paw inflammation with complete Freund's adjuvant. Recordings were made from functionally identified ON, OFF and NEUTRAL cells in the RVM. Spontaneous activity and responses evoked by a series of mechanical (10, 15, 26, 60, 100, and 180 g) and heat (34-50 °C) stimuli applied to the inflamed and non-inflamed hind paws were determined before and at 15 and 60 min after injection of the NK-1-antagonist L-733,060 or vehicle into the RVM. Prolonged inflammation did not alter the proportions of functionally-identified ON, OFF and NEUTRAL cells. ON cells exhibited enhanced responses to mechanical (60-100g) and heat (48-50 °C) stimuli applied to the inflamed paw, which were attenuated by L-733,060 but not by vehicle. Inhibitory responses of OFF cells evoked by mechanical stimuli applied to the inflamed paw were also inhibited by L-733,060, but responses evoked by stimulation of the contralateral paw were increased. Heat-evoked responses of OFF cells were not altered by L-733,060. Also, neither L-733,060 nor vehicle altered spontaneous ongoing discharge rate of RVM neurons. These data indicate that NK-1 receptors modulate excitability of ON cells which contribute to both mechanical and heat hyperalgesia, whereas NK-1 modulation of OFF cells contributes to mechanical hyperalgesia during prolonged inflammation.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Hyperalgesia and sensitization of dorsal horn neurons following activation of NK-1 receptors in the rostral ventromedial medulla.J Neurophysiol. 2017 Nov 1;118(5):2727-2744. doi: 10.1152/jn.00478.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794197 Free PMC article.
-
Increased neuronal expression of neurokinin-1 receptor and stimulus-evoked internalization of the receptor in the rostral ventromedial medulla of the rat after peripheral inflammatory injury.J Comp Neurol. 2014 Sep 1;522(13):3037-51. doi: 10.1002/cne.23564. J Comp Neurol. 2014. PMID: 24639151 Free PMC article.
-
Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors.J Neurophysiol. 2012 Feb;107(4):1210-21. doi: 10.1152/jn.00678.2011. Epub 2011 Oct 26. J Neurophysiol. 2012. PMID: 22031765 Free PMC article.
-
Loss of neurons in rostral ventromedial medulla that express neurokinin-1 receptors decreases the development of hyperalgesia.Neuroscience. 2013 Oct 10;250:151-65. doi: 10.1016/j.neuroscience.2013.06.057. Epub 2013 Jul 3. Neuroscience. 2013. PMID: 23831426 Free PMC article.
-
NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin.Pain. 2008 Sep 30;139(1):34-46. doi: 10.1016/j.pain.2008.02.032. Epub 2008 Apr 14. Pain. 2008. PMID: 18407414
Cited by
-
Bulbospinal nociceptive ON and OFF cells related neural circuits and transmitters.Front Pharmacol. 2023 Apr 20;14:1159753. doi: 10.3389/fphar.2023.1159753. eCollection 2023. Front Pharmacol. 2023. PMID: 37153792 Free PMC article. Review.
-
Regular physical activity reduces the percentage of spinally projecting neurons that express mu-opioid receptors from the rostral ventromedial medulla in mice.Pain Rep. 2020 Dec 2;5(6):e857. doi: 10.1097/PR9.0000000000000857. eCollection 2020 Nov-Dec. Pain Rep. 2020. PMID: 33294758 Free PMC article.
-
Hyperalgesia and sensitization of dorsal horn neurons following activation of NK-1 receptors in the rostral ventromedial medulla.J Neurophysiol. 2017 Nov 1;118(5):2727-2744. doi: 10.1152/jn.00478.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794197 Free PMC article.
-
Neuroplasticity of ascending and descending pathways after somatosensory system injury: reviewing knowledge to identify neuropathic pain therapeutic targets.Spinal Cord. 2016 May;54(5):330-40. doi: 10.1038/sc.2015.225. Epub 2016 Jan 12. Spinal Cord. 2016. PMID: 26754470 Review.
-
Increased neuronal expression of neurokinin-1 receptor and stimulus-evoked internalization of the receptor in the rostral ventromedial medulla of the rat after peripheral inflammatory injury.J Comp Neurol. 2014 Sep 1;522(13):3037-51. doi: 10.1002/cne.23564. J Comp Neurol. 2014. PMID: 24639151 Free PMC article.
References
-
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. - PubMed
-
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

