The role of extracellular signal-related kinase during abdominal aortic aneurysm formation
- PMID: 22917644
- PMCID: PMC3586428
- DOI: 10.1016/j.jamcollsurg.2012.06.414
The role of extracellular signal-related kinase during abdominal aortic aneurysm formation
Abstract
Background: It is hypothesized that activation of extracellular signal-related kinase (ERK) is critical in activating matrix metalloproteinases (MMPs) during abdominal aortic aneurysm (AAA) formation.
Study design: C57BL/6 male mice underwent either elastase or heat-inactivated elastase aortic perfusion (n = 9 per group). Mouse aortic smooth muscle cells were transfected with ERK-1 and 2 siRNA along with or without elastase treatment. Mouse and human aortic tissue were analyzed by Western blots, zymograms, and immunohistochemistry, and statistical analysis was done using Graphpad and Image J softwares.
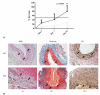
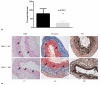
Results: Western blot and immunohistochemistry documented increased phospho-mitogen-activated protein kinase kinase-1/2 (pMEK-1/2; 153%, p = 0.270 by Western) and pERK (171%, p = 0.004 by Western blot) in the elastase perfused aortas. Male ERK-1(-/-) mice underwent elastase perfusion, and aortic diameter was determined at day 14. ERK-1(-/-) mice failed to develop AAA, and histologic analysis depicted intact collagen and elastin fibers in the aortas. Zymography of aortas of elastase-treated ERK-1(-/-) mice showed lower levels of proMMP2 (p < 0.005) and active MMP2 (p < 0.0001), as well as proMMP9 (p = 0.037) compared with C57BL/6 mice. siRNA transfection of ERK-1 and -2 significantly reduced formation of pro- and active MMP2 (p < 0.01 for both isoforms) in aortic smooth muscle cells treated with elastase in vitro. Human AAA tissue had significantly elevated levels of pMEK-1/2 (150%, p = 0.014) and pERK (159%, p = 0.013) compared with control tissues.
Conclusions: The MAPK (mitogen-activated protein kinase)/ERK pathway is an important modulator of MMPs during AAA formation. Targeting the ERK pathway by reagents that inhibit either the expression or phosphorylation of ERK isoforms could be a potential therapy to prevent AAA formation.
Copyright © 2012 American College of Surgeons. Published by Elsevier Inc. All rights reserved.
Figures




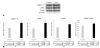
References
-
- Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: Interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann N Y Acad Sci. 2006;1085:117–132. - PubMed
-
- Petersen E, Gineitis A, Wagberg F, Angquist KA. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur J Vasc Endovasc Surg. 2000;20:457–461. - PubMed
-
- Eagleton MJ, Ballard N, Lynch E, et al. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during Angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345–351. - PubMed
-
- Wilson WR, Anderton M, Schwalbe EC, et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113:438–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

