WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011
- PMID: 22917957
- PMCID: PMC6061925
- DOI: 10.1016/j.vaccine.2012.07.089
WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011
Abstract
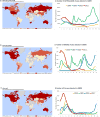
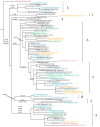
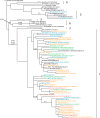
In February and September each year the World Health Organisation (WHO) recommends influenza viruses to be included in influenza vaccines for the forthcoming winters in the Northern and Southern Hemispheres respectively. These recommendations are based on data collected by National Influenza Centres (NIC) through the Global Influenza Surveillance and Response System (GISRS) and a more detailed analysis of representative and potential antigenically variant influenza viruses from the WHO Collaborating Centres for Influenza (WHO CCs) and Essential Regulatory Laboratories (ERLs). This article provides a detailed summary of the antigenic and genetic properties of viruses and additional background data used by WHO experts during development of the recommendations for the 2012 Southern Hemisphere influenza vaccine composition.
Published by Elsevier Ltd.
Figures

References
-
- Barr IG, McCauley J, Cox N, Daniels R, Engelhardt OG, Fukuda K, et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine. 2010 Feb 3;28(5):1156–67. - PubMed
-
- Organization WH. Recommended composition of influenza virus vaccines for use in the 2011–2012 northern hemisphere influenza season. Weekly epidemiological record. 2011;86(10):81–92. - PubMed
-
- Organization WH. Recommended composition of influenza vaccines for use in the 2012 southern hemisphere influenza season. Weekly epidemiological record. 2011;86:457–68. - PubMed
-
- Organization WH. Standardization of terminology of the pandemic A(H1N1)2009 virus. 2011 10/18/2011 [cited 2011 11/01/2011]; Available from: http://www.who.int/influenza/gisrs_laboratory/terminology_ah1n1pdm09/en/ - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

