Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether-lumefantrine in healthy volunteers
- PMID: 22918158
- PMCID: PMC3511816
- DOI: 10.1097/QAI.0b013e31826ebb5c
Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether-lumefantrine in healthy volunteers
Abstract
Background: The antiretroviral drug efavirenz (EFV) and the antimalarial artemisinin-based combination therapy artemether-lumefantrine (AL) are commonly co-administered to treat HIV and malaria. EFV is a known inducer of cytochrome P450 3A4, which converts artemether to dihydroartemisinin (DHA) that is also active and metabolizes longer acting lumefantrine (LR). A study in healthy volunteers was completed to address the concern that EFV impacts AL pharmacokinetics (PKs).
Methods: Adults received AL (80/480 mg twice daily) for 3-days before and during EFV co-administration (600 mg daily for 26 days) with intensive PK for artemether, DHA, and LR conducted after the last AL dose for each period. EFV PK was evaluated with and without AL. PK parameters were estimated using noncompartmental methods.
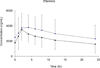
Results: Twelve subjects completed the 2-period study. PK exposure for artemether, DHA, and LR [as estimated by the area under the concentration time curve (AUClast)] decreased or trended toward decrease with EFV, compared with when administered alone [-51% (P = 0.084), -46% (P = 0.005), and -21% (P = 0.102), respectively]. Day-7 LR levels, previously deemed predictive of treatment success, were 46% lower (P = 0.002) with EFV, but the LR half-life was unchanged. EFV PK exposure was minimally altered after AL co-administration [AUC0-24 hrs decreased by 17% (P = 0.034)].
Conclusions: Exposure to DHA, but not LR, was significantly lower during EFV-AL co-administration compared with that during administration of AL alone. These findings may have implications for the treatment efficacy of AL, particularly in children. However, the observed modest changes probably do not warrant dosage adjustment during co-administration of AL with EFV.
Conflict of interest statement
No other conflicts reported.
Figures

Similar articles
-
Extended Treatment Duration of Artemether-Lumefantrine in Ugandan Children with HIV on Efavirenz-Based Antiretroviral Therapy: A Randomized Controlled Pharmacokinetic and Pharmacodynamic Trial.J Clin Pharmacol. 2025 Jul;65(7):909-922. doi: 10.1002/jcph.6193. Epub 2025 Jan 24. J Clin Pharmacol. 2025. PMID: 39853752 Clinical Trial.
-
Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults.J Antimicrob Chemother. 2012 Sep;67(9):2213-21. doi: 10.1093/jac/dks207. Epub 2012 Jun 11. J Antimicrob Chemother. 2012. PMID: 22687893 Free PMC article. Clinical Trial.
-
Efavirenz-Based Antiretroviral Therapy Reduces Artemether-Lumefantrine Exposure for Malaria Treatment in HIV-Infected Pregnant Women.J Acquir Immune Defic Syndr. 2020 Feb 1;83(2):140-147. doi: 10.1097/QAI.0000000000002237. J Acquir Immune Defic Syndr. 2020. PMID: 31929402 Free PMC article.
-
Understanding the pharmacokinetics of Coartem.Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. Malar J. 2009. PMID: 19818171 Free PMC article. Review.
-
Artemether-lumefantrine: an option for malaria.Ann Pharmacother. 2012 Apr;46(4):567-77. doi: 10.1345/aph.1Q539. Epub 2012 Apr 10. Ann Pharmacother. 2012. PMID: 22496476 Review.
Cited by
-
CYP2B6*6 genotype and high efavirenz plasma concentration but not nevirapine are associated with low lumefantrine plasma exposure and poor treatment response in HIV-malaria-coinfected patients.Pharmacogenomics J. 2016 Feb;16(1):88-95. doi: 10.1038/tpj.2015.37. Epub 2015 May 12. Pharmacogenomics J. 2016. PMID: 25963334
-
Health Considerations for HIV-Infected International Travelers.Curr Infect Dis Rep. 2019 Apr 12;21(5):16. doi: 10.1007/s11908-019-0672-y. Curr Infect Dis Rep. 2019. PMID: 30980287 Review.
-
Development of Guanfacine Extended-Release Dosing Strategies in Children and Adolescents with ADHD Using a Physiologically Based Pharmacokinetic Model to Predict Drug-Drug Interactions with Moderate CYP3A4 Inhibitors or Inducers.Paediatr Drugs. 2018 Apr;20(2):181-194. doi: 10.1007/s40272-017-0270-0. Paediatr Drugs. 2018. PMID: 29098603 Free PMC article.
-
Development of an evidence evaluation and synthesis system for drug-drug interactions, and its application to a systematic review of HIV and malaria co-infection.PLoS One. 2017 Mar 23;12(3):e0173509. doi: 10.1371/journal.pone.0173509. eCollection 2017. PLoS One. 2017. PMID: 28334018 Free PMC article.
-
Efficacy and safety of artemether-lumefantrine as treatment for Plasmodium falciparum uncomplicated malaria in adult patients on efavirenz-based antiretroviral therapy in Zambia: an open label non-randomized interventional trial.Malar J. 2019 May 24;18(1):180. doi: 10.1186/s12936-019-2818-7. Malar J. 2019. PMID: 31126288 Free PMC article. Clinical Trial.
References
-
- Centers for Disease Control and Prevention. Malaria biology. Available at: http://www.cdc.gov/malaria/about/biology/index.html.
-
- World Health Organization. World Malaria Report 2011. Available at: http://www.who.int/malaria/world_malaria_report_2011/en/index.html.
-
- World Health Organization. Global epidemic, HIV/AIDS, data and statistics. Available as powerpoint slides at: http://www.who.int/hiv/data/en/.
-
- Rogerson SR, Gladstone M, Callaghan M, et al. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans. R. Soc Trop Med Hyg. 2004;98:544–552. - PubMed
-
- Kamya MR, Kigonya CN, McFarland W. HIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in children. AIDS. 2001;15(9):1187–1188. - PubMed

