RNA recognition by double-stranded RNA binding domains: a matter of shape and sequence
- PMID: 22918483
- PMCID: PMC3724394
- DOI: 10.1007/s00018-012-1119-x
RNA recognition by double-stranded RNA binding domains: a matter of shape and sequence
Abstract
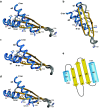
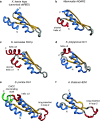
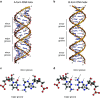
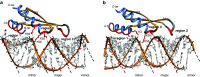
The double-stranded RNA binding domain (dsRBD) is a small protein domain of 65-70 amino acids adopting an αβββα fold, whose central property is to bind to double-stranded RNA (dsRNA). This domain is present in proteins implicated in many aspects of cellular life, including antiviral response, RNA editing, RNA processing, RNA transport and, last but not least, RNA silencing. Even though proteins containing dsRBDs can bind to very specific dsRNA targets in vivo, the binding of dsRBDs to dsRNA is commonly believed to be shape-dependent rather than sequence-specific. Interestingly, recent structural information on dsRNA recognition by dsRBDs opens the possibility that this domain performs a direct readout of RNA sequence in the minor groove, allowing a global reconsideration of the principles describing dsRNA recognition by dsRBDs. We review in this article the current structural and molecular knowledge on dsRBDs, emphasizing the intricate relationship between the amino acid sequence, the structure of the domain and its RNA recognition capacity. We especially focus on the molecular determinants of dsRNA recognition and describe how sequence discrimination can be achieved by this type of domain.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

