A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids
- PMID: 22918645
- PMCID: PMC3465014
- DOI: 10.1167/iovs.12-10279
A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids
Abstract
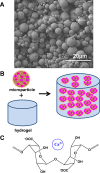
Purpose: To design and develop a drug-delivery system containing a combination of poly(D,L-lactide-co-glycolide) (PLGA) microparticles and alginate hydrogel for sustained release of retinoids to treat retinal blinding diseases that result from an inadequate supply of retinol and generation of 11-cis-retinal.
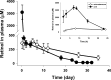
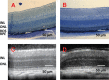
Methods: To study drug release in vivo, either the drug-loaded microparticle-hydrogel combination was injected subcutaneously or drug-loaded microparticles were injected intravitreally into Lrat(-/-) mice. Orally administered 9-cis-retinoids were used for comparison and drug concentrations in plasma were determined by HPLC. Electroretinography (ERG) and both chemical and histologic analyses were used to evaluate drug effects on visual function and morphology.
Results: Lrat(-/-) mice demonstrated sustained drug release from the microparticle/hydrogel combination that lasted 4 weeks after subcutaneous injection. Drug concentrations in plasma of the control group treated with the same oral dose rose to higher levels for 6-7 hours but then dropped markedly by 24 hours. Significantly increased ERG responses and a markedly improved retinal pigmented epithelium (RPE)-rod outer segment (ROS) interface were observed after subcutaneous injection of the drug-loaded delivery combination. Intravitreal injection of just 2% of the systemic dose of drug-loaded microparticles provided comparable therapeutic efficacy.
Conclusions: Sustained release of therapeutic levels of 9-cis-retinoids was achieved in Lrat(-/-) mice by subcutaneous injection in a microparticle/hydrogel drug-delivery system. Both subcutaneous and intravitreal injections of drug-loaded microparticles into Lrat(-/-) mice improved visual function and retinal structure.
Conflict of interest statement
Disclosure:
Figures



Similar articles
-
Co-delivery of vascular endothelial growth factor and angiopoietin-1 using injectable microsphere/hydrogel hybrid systems for therapeutic angiogenesis.Pharm Res. 2013 Aug;30(8):2157-65. doi: 10.1007/s11095-013-1076-6. Epub 2013 May 18. Pharm Res. 2013. PMID: 23686375
-
Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system.Pharm Res. 2009 Jul;26(7):1739-44. doi: 10.1007/s11095-009-9884-4. Epub 2009 Apr 21. Pharm Res. 2009. PMID: 19384466
-
Effective and sustained delivery of hydrophobic retinoids to photoreceptors.Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5958-64. doi: 10.1167/iovs.10-5766. Epub 2010 Jun 23. Invest Ophthalmol Vis Sci. 2010. PMID: 20574023 Free PMC article.
-
Fabrication and Use of Poly(d,l-lactide-co-glycolide)-Based Formulations Designed for Modified Release of 5-Fluorouracil.J Pharm Sci. 2018 Feb;107(2):513-528. doi: 10.1016/j.xphs.2017.10.012. Epub 2017 Oct 16. J Pharm Sci. 2018. PMID: 29045885 Free PMC article. Review.
-
Retinoids for treatment of retinal diseases.Trends Pharmacol Sci. 2010 Jun;31(6):284-95. doi: 10.1016/j.tips.2010.03.001. Trends Pharmacol Sci. 2010. PMID: 20435355 Free PMC article. Review.
Cited by
-
Intervisit variability of visual parameters in Leber congenital amaurosis caused by RPE65 mutations.Invest Ophthalmol Vis Sci. 2013 Feb 15;54(2):1378-83. doi: 10.1167/iovs.12-11341. Invest Ophthalmol Vis Sci. 2013. PMID: 23341016 Free PMC article.
-
Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach.Nanomaterials (Basel). 2019 Jun 14;9(6):884. doi: 10.3390/nano9060884. Nanomaterials (Basel). 2019. PMID: 31207951 Free PMC article. Review.
-
Vitamin A derivatives as treatment options for retinal degenerative diseases.Nutrients. 2013 Jul 12;5(7):2646-66. doi: 10.3390/nu5072646. Nutrients. 2013. PMID: 23857173 Free PMC article. Review.
-
Integration of drug, protein, and gene delivery systems with regenerative medicine.Drug Deliv Transl Res. 2015 Apr;5(2):168-86. doi: 10.1007/s13346-013-0165-8. Drug Deliv Transl Res. 2015. PMID: 25787742 Free PMC article. Review.
-
Drug-eluting nasal implants: formulation, characterization, clinical applications and challenges.Pharmaceutics. 2014 May 27;6(2):249-67. doi: 10.3390/pharmaceutics6020249. Pharmaceutics. 2014. PMID: 24871904 Free PMC article.
References
-
- Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

