Platelet signaling
- PMID: 22918727
- PMCID: PMC4785017
- DOI: 10.1007/978-3-642-29423-5_3
Platelet signaling
Abstract
This chapter summarizes current ideas about the intracellular signaling that drives platelet responses to vascular injury. After a brief overview of platelet activation intended to place the signaling pathways into context, the first section considers the early events of platelet activation leading up to integrin activation and platelet aggregation. The focus is on the G protein-mediated events utilized by agonists such as thrombin and ADP, and the tyrosine kinase-based signaling triggered by collagen. The second section considers the events that occur after integrin engagement, some of which are dependent on close physical contact between platelets. A third section addresses the regulatory events that help to avoid unprovoked or excessive platelet activation, after which the final section briefly considers individual variations in platelet reactivity and the role of platelet signaling in the innate immune response and embryonic development.
Figures



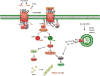
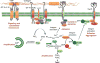

References
-
- Barr AJ, Brass LF, Manning DR. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells: a direct evaluation of selectivity in receptor-G protein coupling. J Biol Chem. 1997;272:2223–2229. - PubMed
-
- Börsch-Haubold AG, Kramer RM, Watson SP. Cytosolic phospholipase A2 is phosphorylated in collagen- and thrombin-stimulated human platelets independent of protein kinase C and mitogen-activated protein kinase. J Biol Chem. 1995;270:25885–25892. - PubMed
-
- Börsch-Haubold AG, Ghomashchi F, Pasquet S, Goedert M, Cohen P, Gelb MH, Watson SP. Phosphorylation of cytosolic phospholipase A2 in platelets is mediated by multiple stress-activated protein kinase pathways. Eur J Biochem. 1999;265:195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

